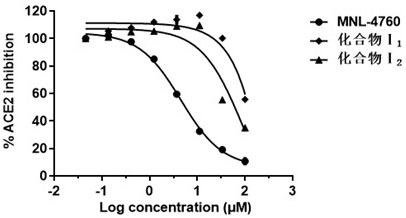
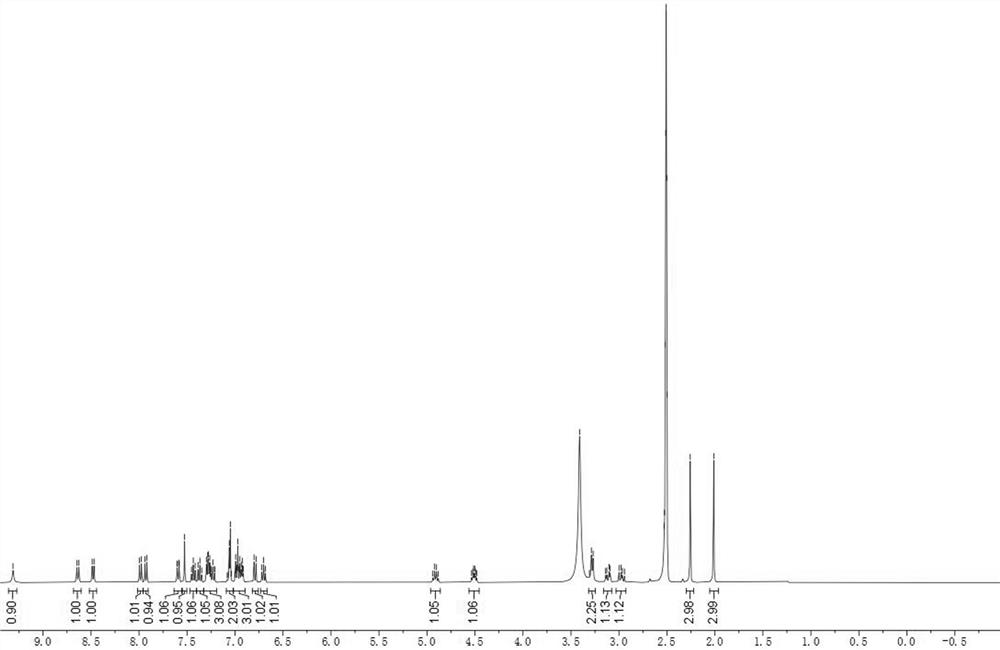
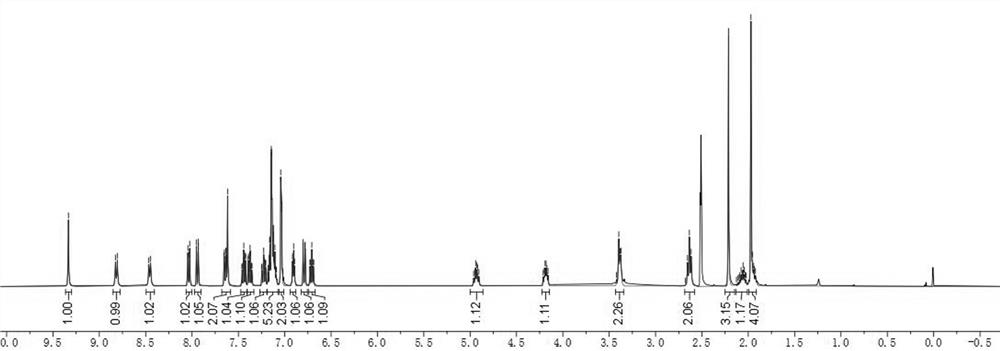
Angiotensin converting enzyme 2 inhibitor, application thereof and anti-coronavirus infection medicine
An angiotensin, coronavirus technology, applied in the field of angiotensin converting enzyme 2 inhibitors, anti-coronavirus infection drugs, can solve the problem of poor activity and pharmacokinetic properties, restricted use, immunogenicity, oral biological Poor utilization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] In some embodiments, the preparation method of angiotensin-converting enzyme 2 inhibitor comprises:
[0055] S01. Provide a solid phase carrier, amino acid substrate I and condensing agent. The amino terminal of amino acid substrate I is connected with an amino protecting group. Dissolve the solid phase carrier and amino acid substrate I in the reaction solvent, add the condensing agent, and then inert gas The reaction is carried out under the environment, so that the amino acid substrate I is immobilized on the solid phase support through the carboxyl terminal connection, and the first resin is obtained;
[0056] S02, removing the amino protecting group on the first resin to obtain the second resin;
[0057] S03. Taking the amino acid substrate I with the amino protecting group removed on the second resin as the synthesis starting point, refer to the operations of steps S01 and S02 to repeat the steps of condensation into peptides, washing, deprotection, and washing, a...
Embodiment 1
[0065] Compound I was synthesized in this example 1 : , including the following steps:
[0066] S11. Dissolve 2-CTC resin (0.10 mmol, 1.00 eq, Sub 1.10 mmol / g) and Fmoc-L-4-fluorophenylalanine (60.7 mg, 0.15 mmol, 1.50 eq) in 3 mL DCM, add dropwise DIEA (51.6 mg, 0.40 mmol, 0.066 mL, 4.00 eq), refluxed at room temperature for 2 h under nitrogen protection, added 0.5 mL of methanol, and filtered the reaction system after 30 min to obtain the first resin. In the first resin, Fmoc-L- 4-Fluorophenylalanine is immobilized on 2-CTC resin through carboxyl-terminal connection;
[0067] S12. Dissolve the first resin in a DMF solution (100 mL) containing 20% piperidine, stir for 5 min under nitrogen protection, wash the resin with DMF (80 mL), and filter to obtain the second resin; compared to the first resin, the The L-4-fluorophenylalanine part on the second resin has taken off the protecting group Fmoc;
[0068] S13. Dissolve HBTU (108 mg, 0.285 mmol, 2.85 eq) and Fmoc-L-3-(3-...
Embodiment 2
[0076] Compound I was synthesized in this example 2 : , including the following steps:
[0077] S21. Dissolve 2-CTC resin (0.10 mmol, 1.00 eq, Sub 1.10 mmol / g) and Fmoc-D-phenylalanine (60.7 mg, 0.15 mmol, 1.50 eq) in 3 mL DCM, add dropwise DIEA ( 51.6 mg, 0.40 mmol, 0.066 mL, 4.00 eq), reflux at room temperature for 2 h under nitrogen protection, add 0.5 mL of methanol, filter the reaction system after 30 min to obtain the first resin, in the first resin, Fmoc-D-phenylbutylene The amino acid is immobilized on the 2-CTC resin through the carboxyl terminal connection;
[0078] S22. Dissolve the first resin in a DMF solution (100 mL) containing 20% piperidine, stir for 5 min under nitrogen protection, wash the resin with DMF (80 mL), and filter to obtain the second resin; compared to the first resin, the The D-phenylalanine part on the second resin has taken off the protecting group Fmoc;
[0079] S23. Dissolve HBTU (108 mg, 0.285 mmol, 2.85 eq) and Fmoc-L-3-(3-benzothien...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com