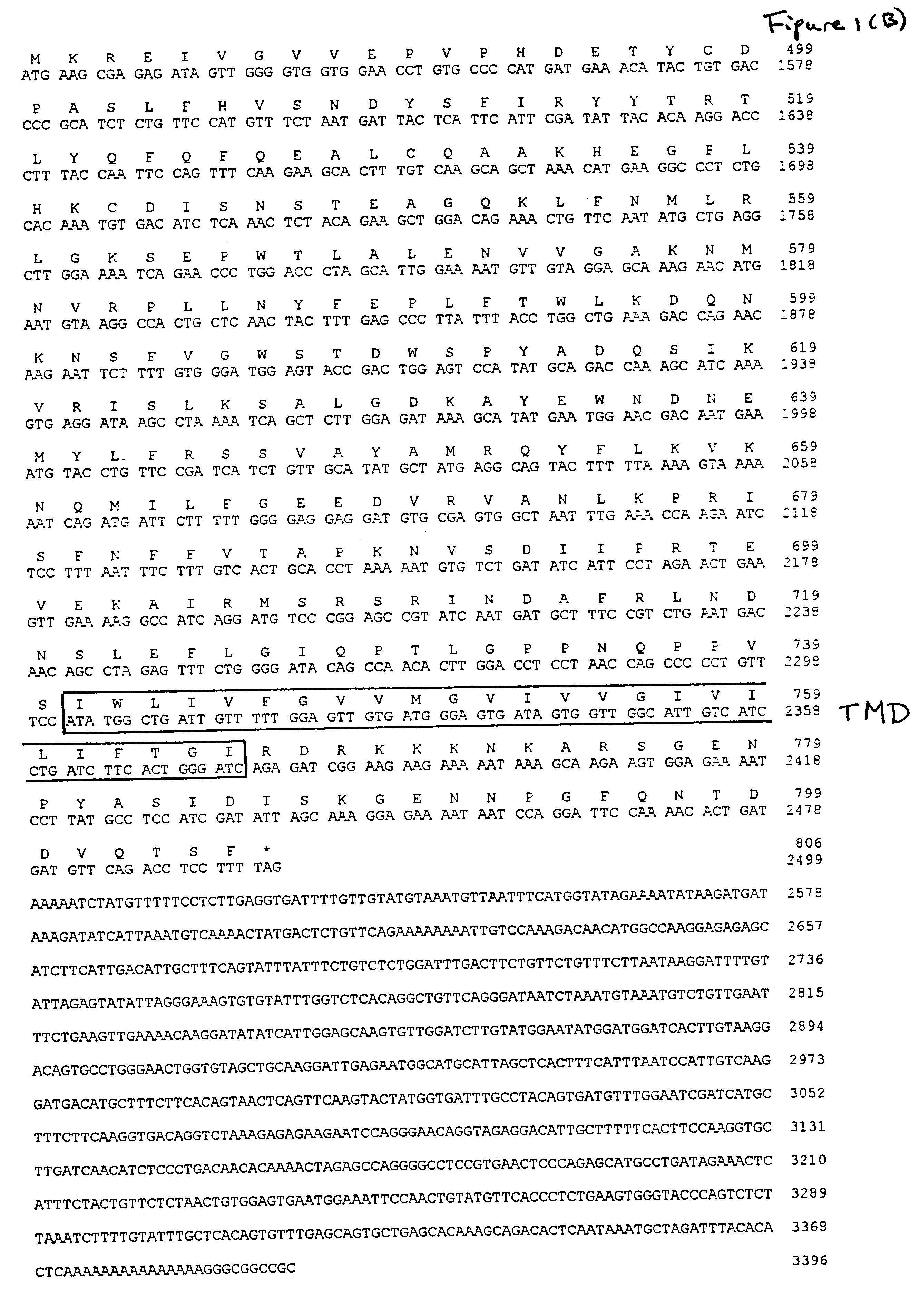
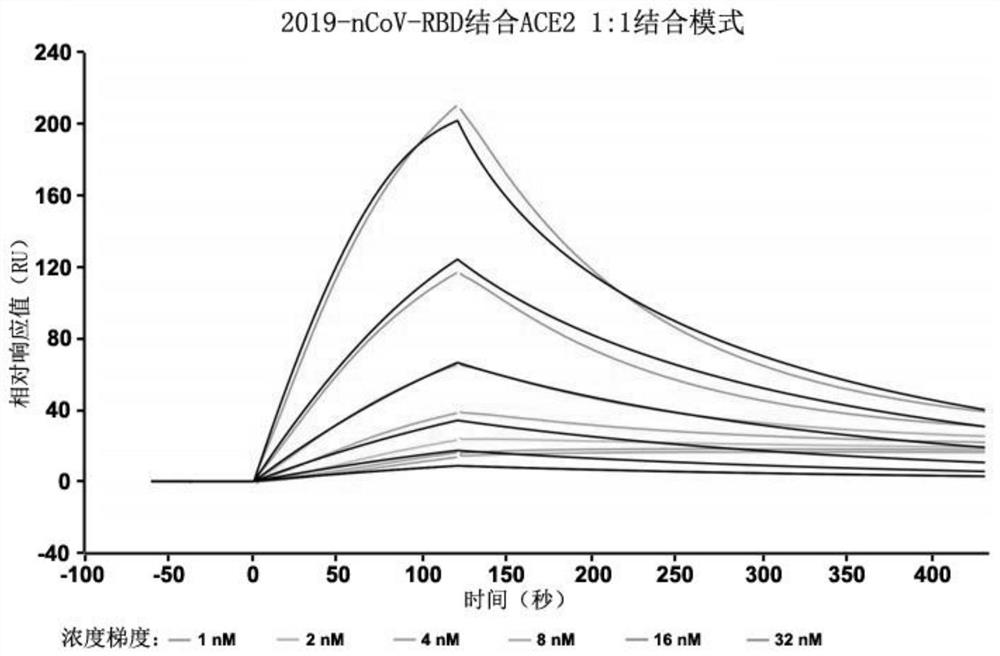
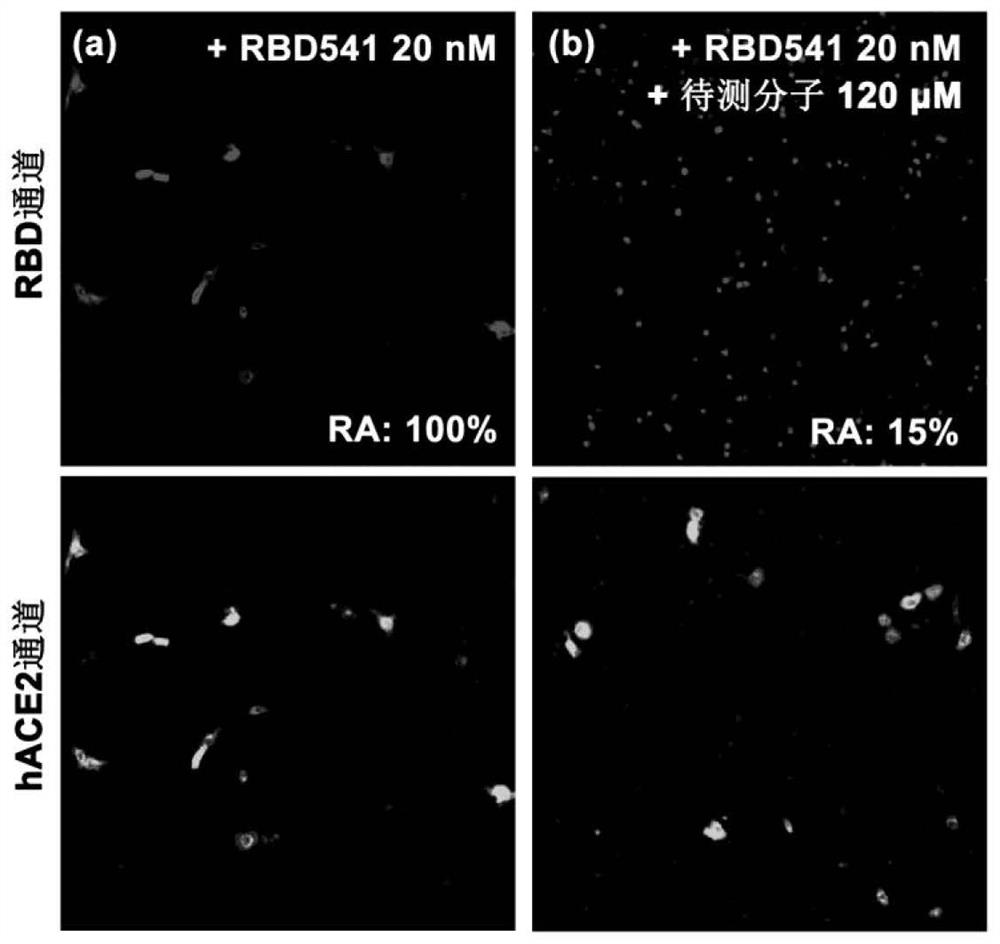
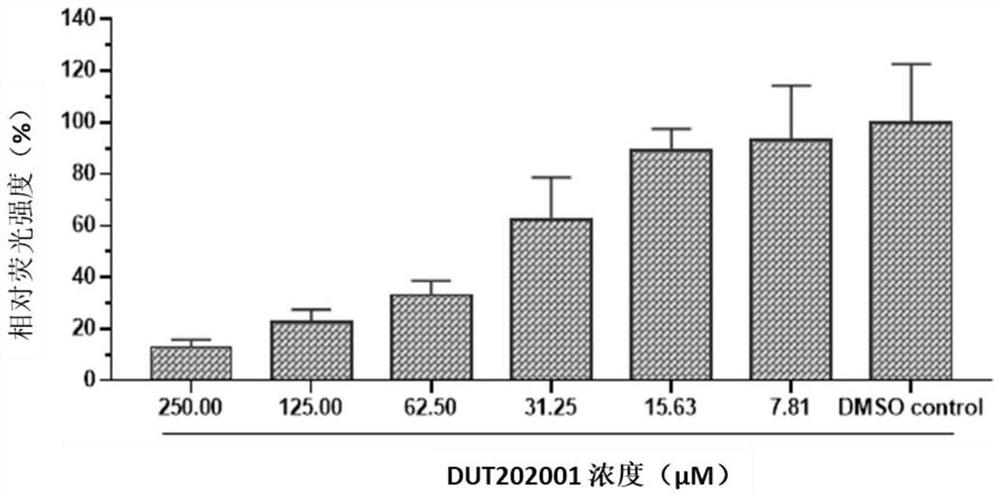
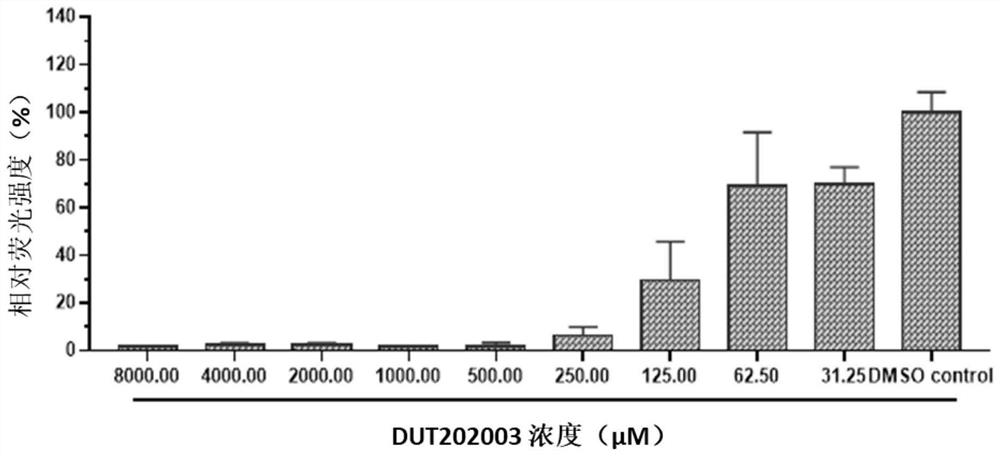
Patents
Literature
46 results about "Angiotensin-converting enzyme 2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
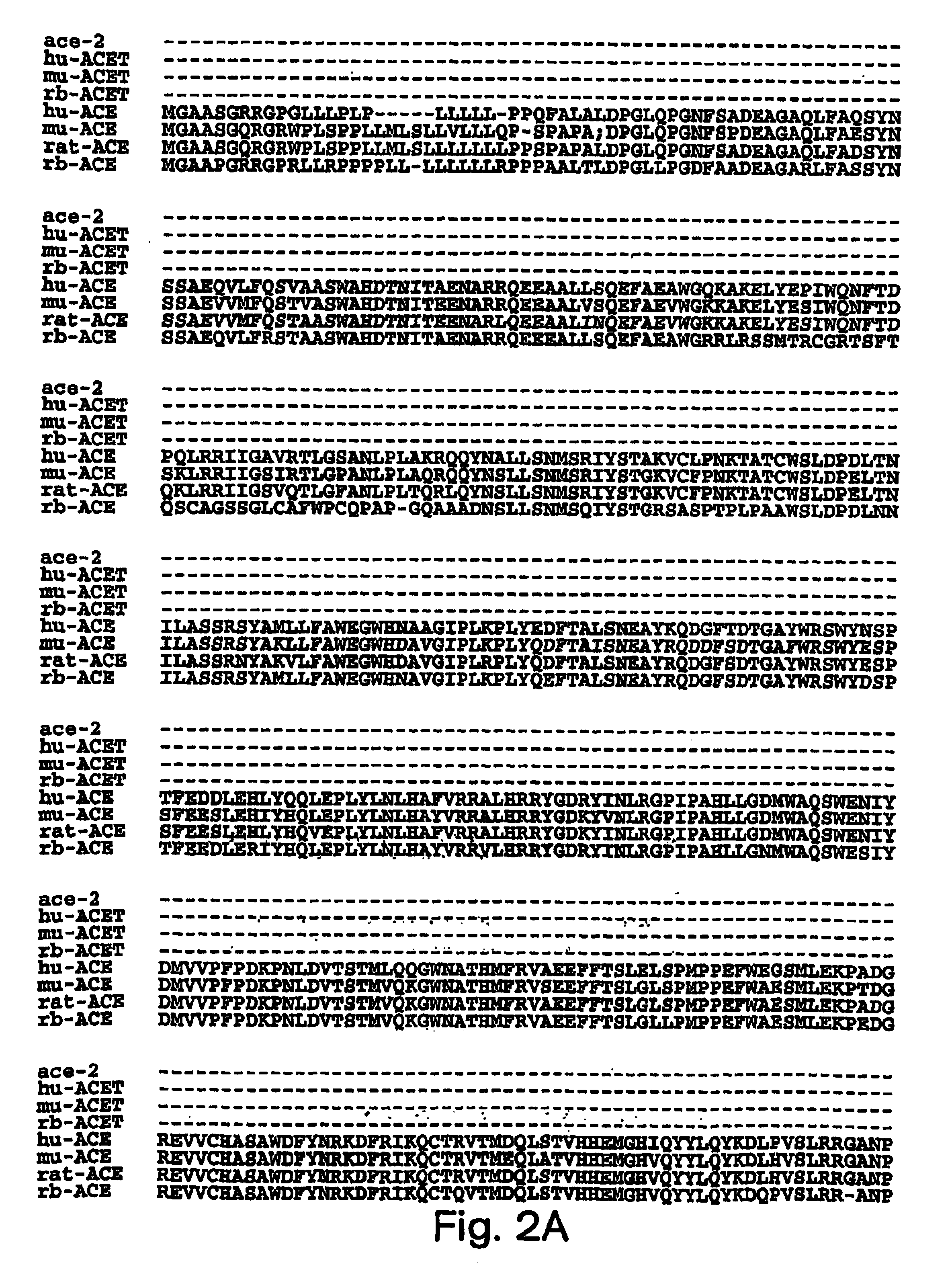
Angiotensin converting enzyme 2 (ACE 2) is an exopeptidase that catalyses the conversion of angiotensin I to the nonapeptide angiotensin[1-9], or the conversion of angiotensin II to angiotensin 1-7. ACE 2 has direct effects on cardiac function, and is expressed predominantly in vascular endothelial cells of the heart and the kidneys.
Angiotensin converting enzyme homolog and therapeutic and diagnostic uses therfor
InactiveUS6194556B1Easy to determineEffectively prescribeSugar derivativesBacteriaAngiotensin-converting enzymeAngiotensin-Converting Enzyme Homolog
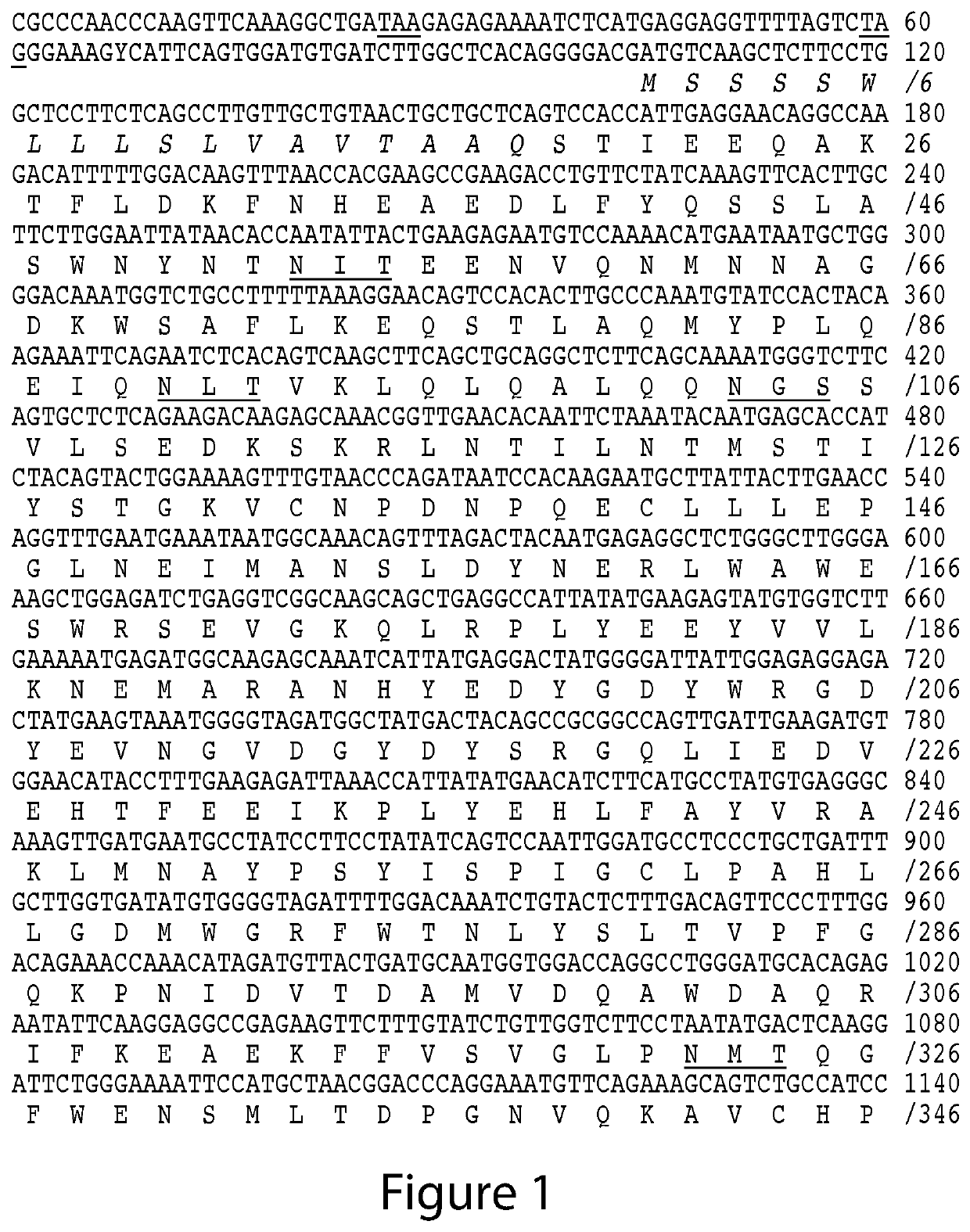
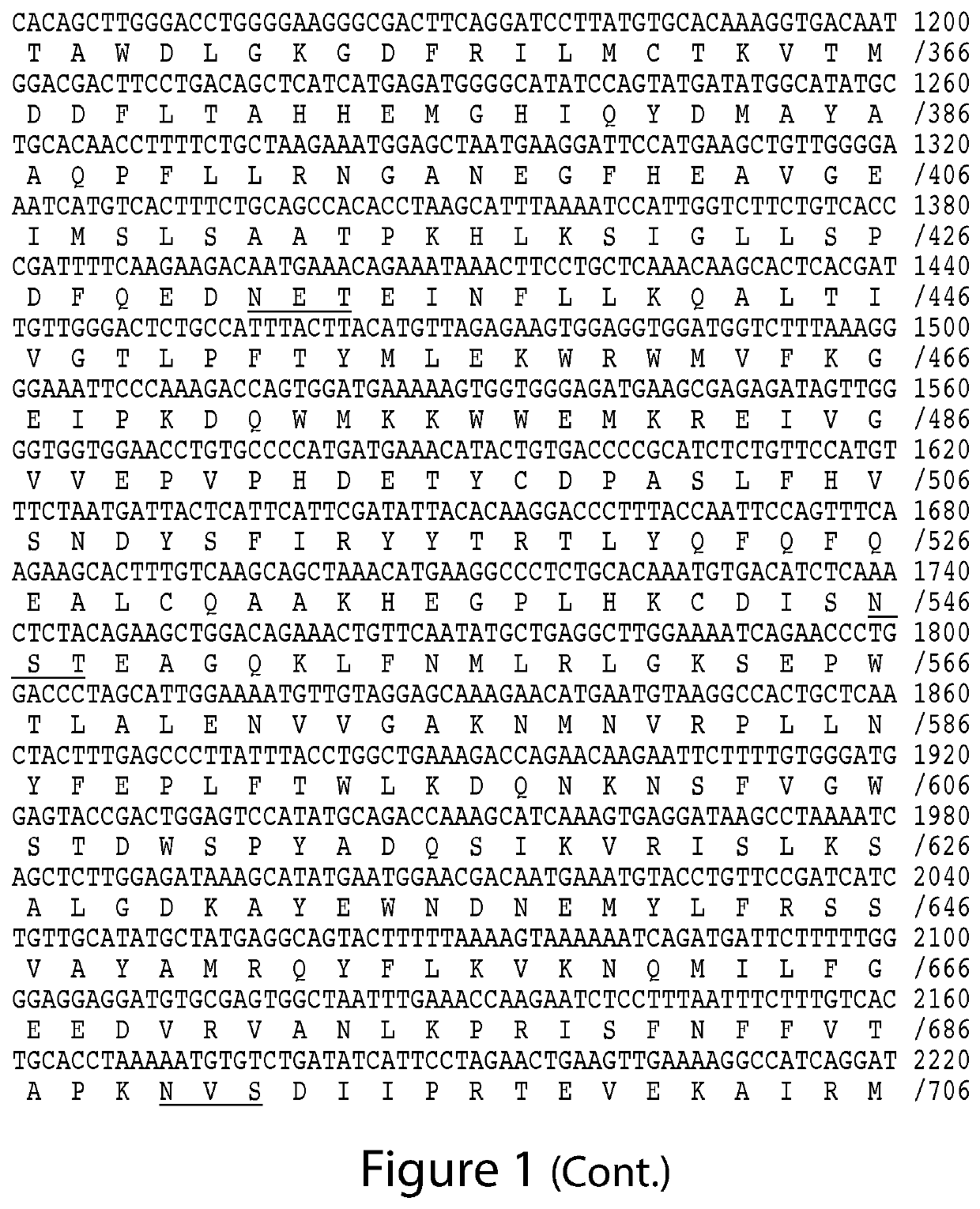
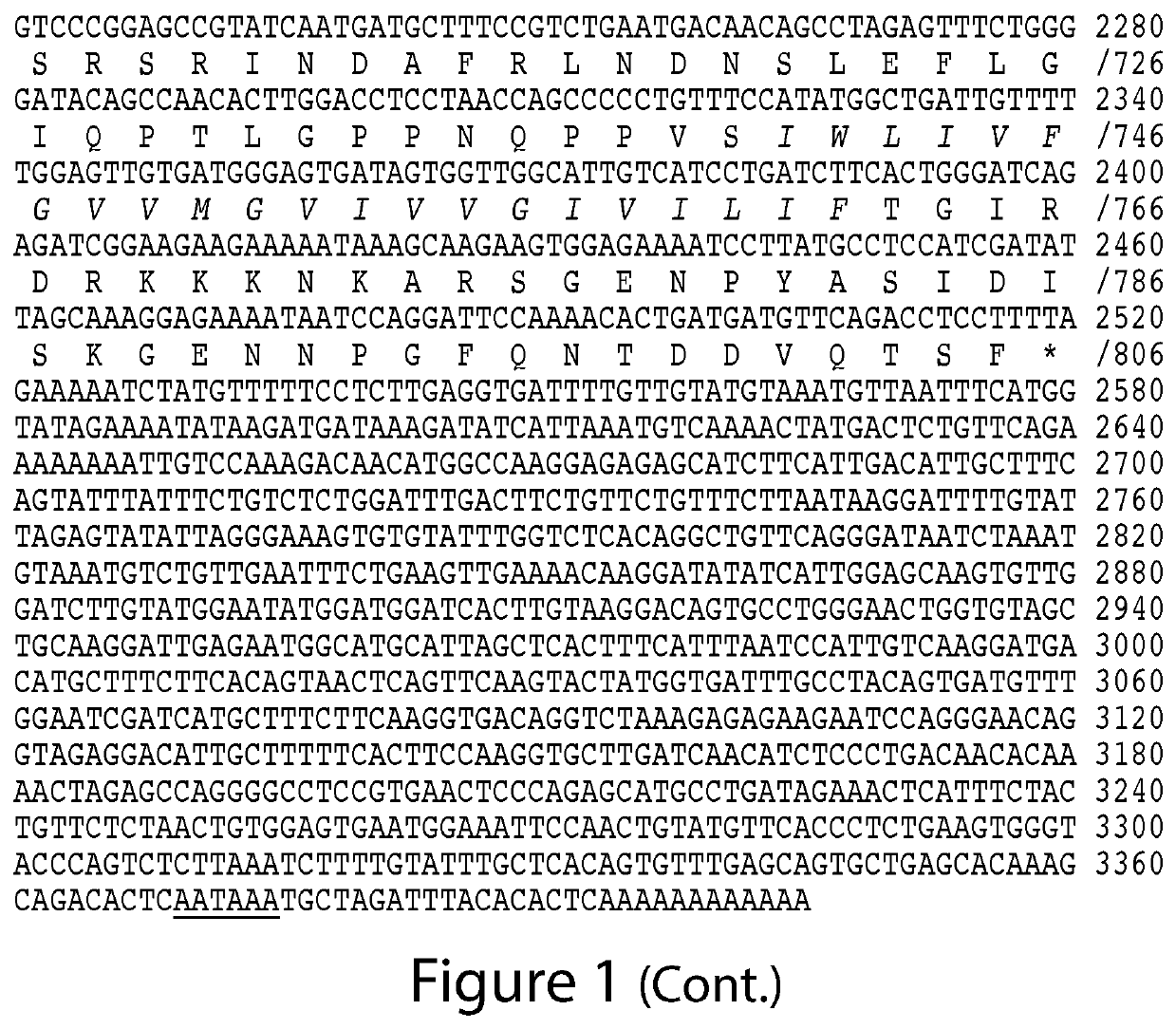
The present invention relates to the discovery of novel genes encoding an angiotensin converting enzyme, Angiotensin Converting Enzyme-2 (ACE-2). Therapeutics, diagnostics and screening assays based on these molecules are also disclosed.
Owner:MILLENNIUM PHARMA INC
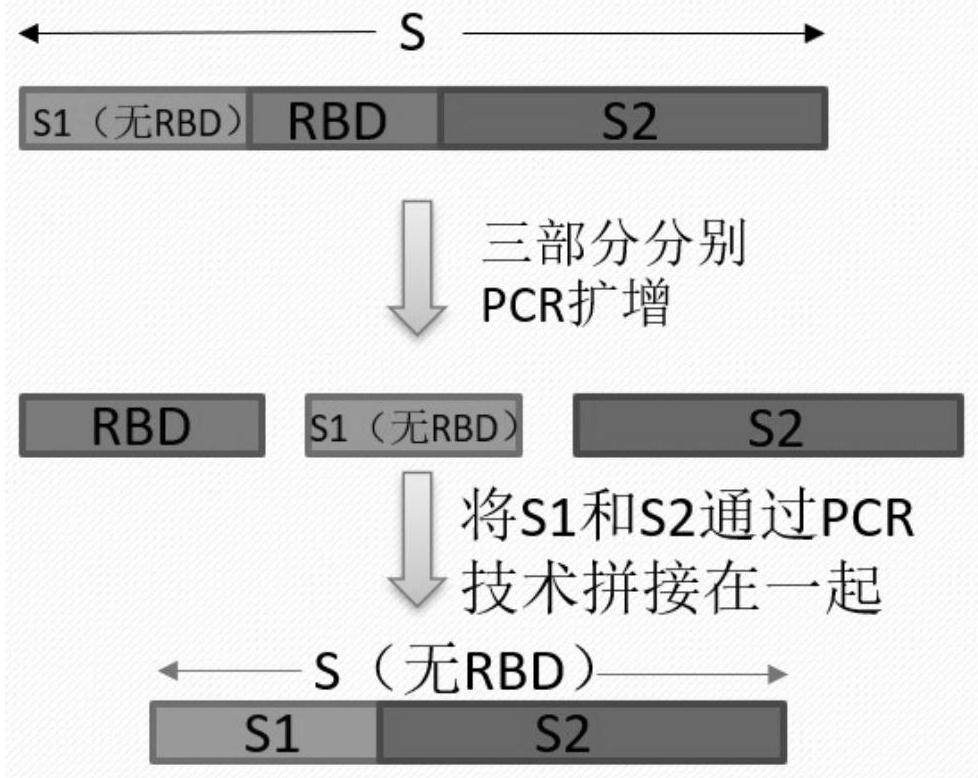
Protein for resisting SARS-CoV-2 infection and vaccine
The invention relates to a protein for resisting SARS-CoV-2 infection and a vaccine, and belongs to the field of medicines. The problem of lack of effective prevention and treatment drugs for SARS-CoV-2 infection is solved. On one hand, the protein for resisting SARS-CoV-2 infection contains a structural domain combined with an angiotensin converting enzyme 2 receptor in an S protein of SARS-CoV-2, and the first amino acid arginine at the N end of the structural domain can be deleted. On the other hand, the vaccine for preventing and / or treating the SARS-CoV-2 infection contains the protein for resisting the SARS-CoV-2 infection, and pharmaceutically acceptable auxiliary materials or auxiliary components. According to the protein for resisting SARS-CoV-2 infection and the vaccine, the combination of the S protein of the SARS-CoV-2 and an ACE2 receptor of a host cell is blocked by inducing in-vivo generation of antibodies and other immune responses, so that a host is helped to resist coronavirus infection.
Owner:WEST VAC BIOPHARMA CO LTD
Colloidal gold immunochromatographic test strip for detecting in-vivo neutralizing antibody after injection of novel coronavirus vaccine and preparation method of colloidal gold immunochromatographic test strip
PendingCN112485436ARapid Qualitative DetectionHigh sensitivityBiological testingImmunoassaysNeutralising antibodyColloidal au
The invention discloses a colloidal gold immunochromatographic test strip for detecting a neutralizing antibody secreted in vivo after injection of a novel coronavirus vaccine and a preparation methodof the colloidal gold immunochromatographic test strip. The test strip comprises a PVC bottom plate, and a sample pad, a colloidal gold pad, a nitrocellulose membrane and water absorption filter paper are sequentially lapped on the PVC bottom plate from left to right; the colloidal gold pad is coated with a recombinant novel coronavirus RBD protein marked by colloidal gold; and the nitrocellulosemembrane is coated with an angiotensin converting enzyme 2 (ACE2) detection line and coated with a mouse anti-chicken IgG antibody as a quality control line. According to the colloidal gold immunochromatography test strip, a competitive method is adopted to detect the neutralizing antibody in a human body, the sensitivity, specificity, repeatability and stability are high, the recovery rate of atarget compound is high, and the detection result is accurate and reliable. The test strip realizes rapid qualitative detection of the neutralizing antibody in a human body after injection of a novelcoronavirus vaccine, has high sensitivity and small intra-batch and inter-batch difference, and provides great convenience for clinical use.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Novel coronavirus SARS-CoV-2 S protein detection method
PendingCN111273006ASolve the problem of inability to effectively detect the new coronavirus SARS-CoV-2S proteinFast preparationImmunoassaysAngiotensin-converting enzymeProtein detection
The invention discloses a novel coronavirus SARS-CoV-2 S protein detection method. SARS-CoV-2 virus depends on spike protein on the surface to be combined with angiotensin converting enzyme 2 on the surface of a cell so as to enter the cell; according to the method, raw materials commonly used in chemiluminescence immunoassay and ACE2 are easy to purchase from the market, the biotin labeling and the alkaline phosphatase labeling are rapid, the reagent for SARS-CoV-2 S protein detection can be rapidly prepared, and the positive coincidence rate, the negative coincidence rate and the total coincidence rate are high compared with the SARS-CoV-2 fluorescence quantitative PCR result.
Owner:SICHUAN ORIENTER BIOLOGICAL TECH
Angiotensin converting enzyme homolog and therapeutic and diagnostic uses therefor
InactiveUS6989363B1Easy to determineEffectively prescribePeptide/protein ingredientsMicroorganism based processesAngiotensin-converting enzymeAllergic condition
The present invention relates to the discovery of novel genes encoding an angiotensin converting enzyme, Angiotensin Converting Enzyme-2 (ACE-2). The invention provides therapeutics, prognostic and diagnostics methods for treating blood pressure related disorders as well as various types of allergic conditions, among others. Also disclosed are screening assays for identifying compounds for treating and preventing these conditions.
Owner:MILLENNIUM PHARMA INC
Immunochromatographic test paper for detecting novel coronavirus
ActiveCN111879933AAccurate identificationAvoid missing detectionMaterial analysisAgainst vector-borne diseasesBlood vesselAffitin
The invention relates to immunochromatographic test paper for detecting novel coronavirus SARS-CoV-2. The test paper comprises a substrate, and a sample pad, a combination pad, a nitrocellulose membrane and a water absorption pad which are arranged on the substrate and are sequentially and fixedly adhered to the substrate along the flowing direction of a to-be-detected liquid sample, the substrateis a PVC plate. The sample pad is coated with a biotin labeled anti-SARS-CoV-2N protein monoclonal antibody and biotin labeled angiotensin converting enzyme 2 (ACE2), the combination pad is coated with avidin crosslinked colloidal gold, the nitrocellulose membrane is provided with a detection line and a quality control line, the detection line is coated with an anti-SARS-CoV-2M protein monoclonalantibody, and the quality control line is coated with goat anti-mouse IgG. The immunochromatographic test paper provided by the invention achieves high-sensitivity, high-specificity, high-speed and convenient-to-operate detection, and can be applied to large-scale rapid screening of people in primary hospitals and communities.
Owner:广州德成生物科技有限公司
Nasal spray preparation for resisting respiratory virus infection
PendingCN111529685AExpand the scope ofImprove adaptabilityOrganic active ingredientsPeptide/protein ingredientsLiposomeSaline
The invention relates to a nasal spray preparation for resisting respiratory virus infection. The preparation comprises liposome-loaded angiotensin converting enzyme 2 (ACE2) recombinant protein, sialic acid, natural polysaccharide and normal saline. The nasal spray preparation is mainly prepared from the liposome-loaded ACE2 extracellular domain recombinant protein, can competitively inhibit thecombination of viruses and host cells, prevents and treats respiratory tract infection caused by various viruses, and enhances the antiviral range and clinical indications of the nasal spray preparation for resisting respiratory tract virus infection. In addition, the nasal spray preparation can be used for directly spraying medicines to virus propagation parts, and is convenient and simple to use.
Owner:XIAMEN NUOKANGDE BIOLOGICAL TECH CO LTD +1
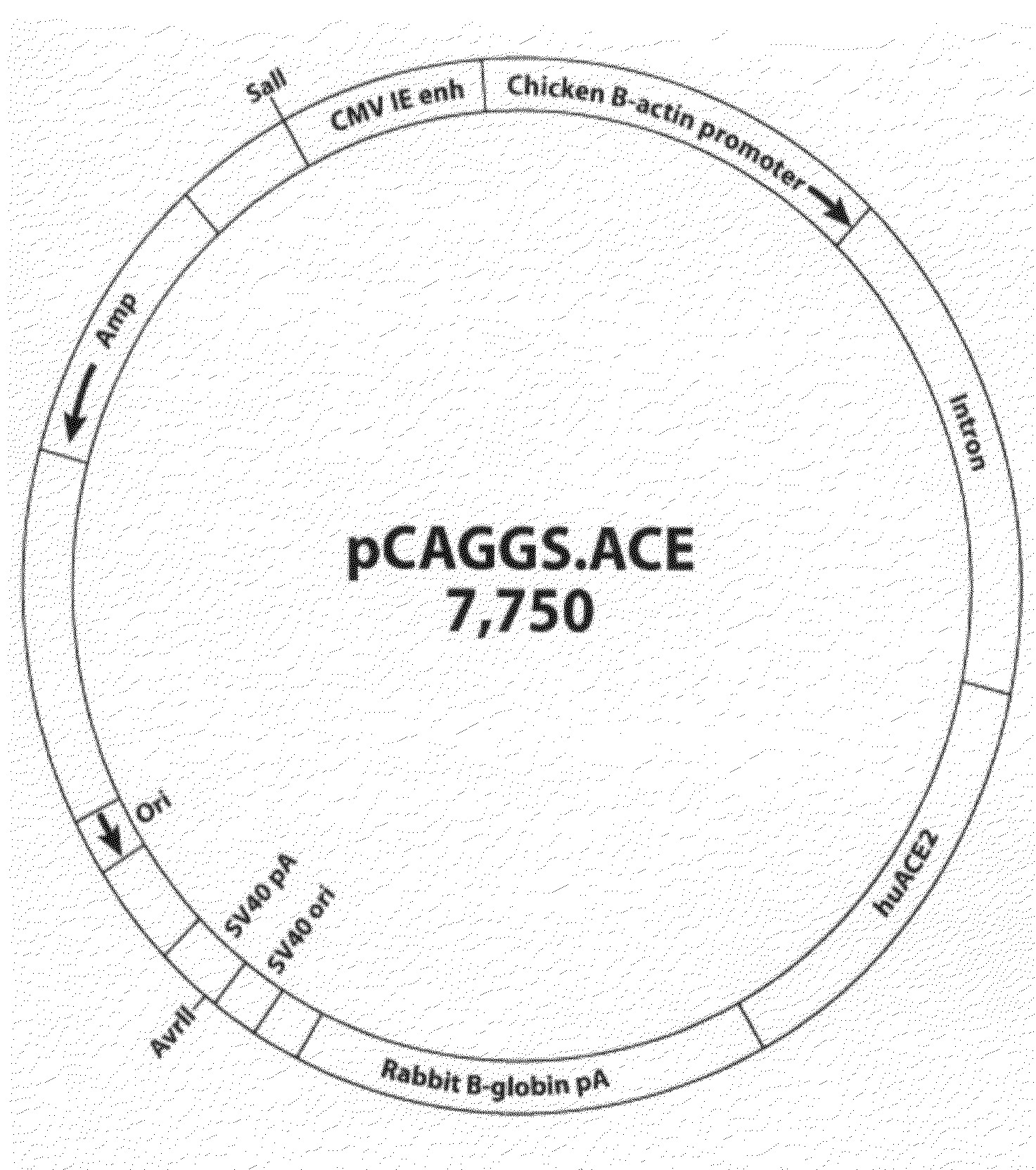
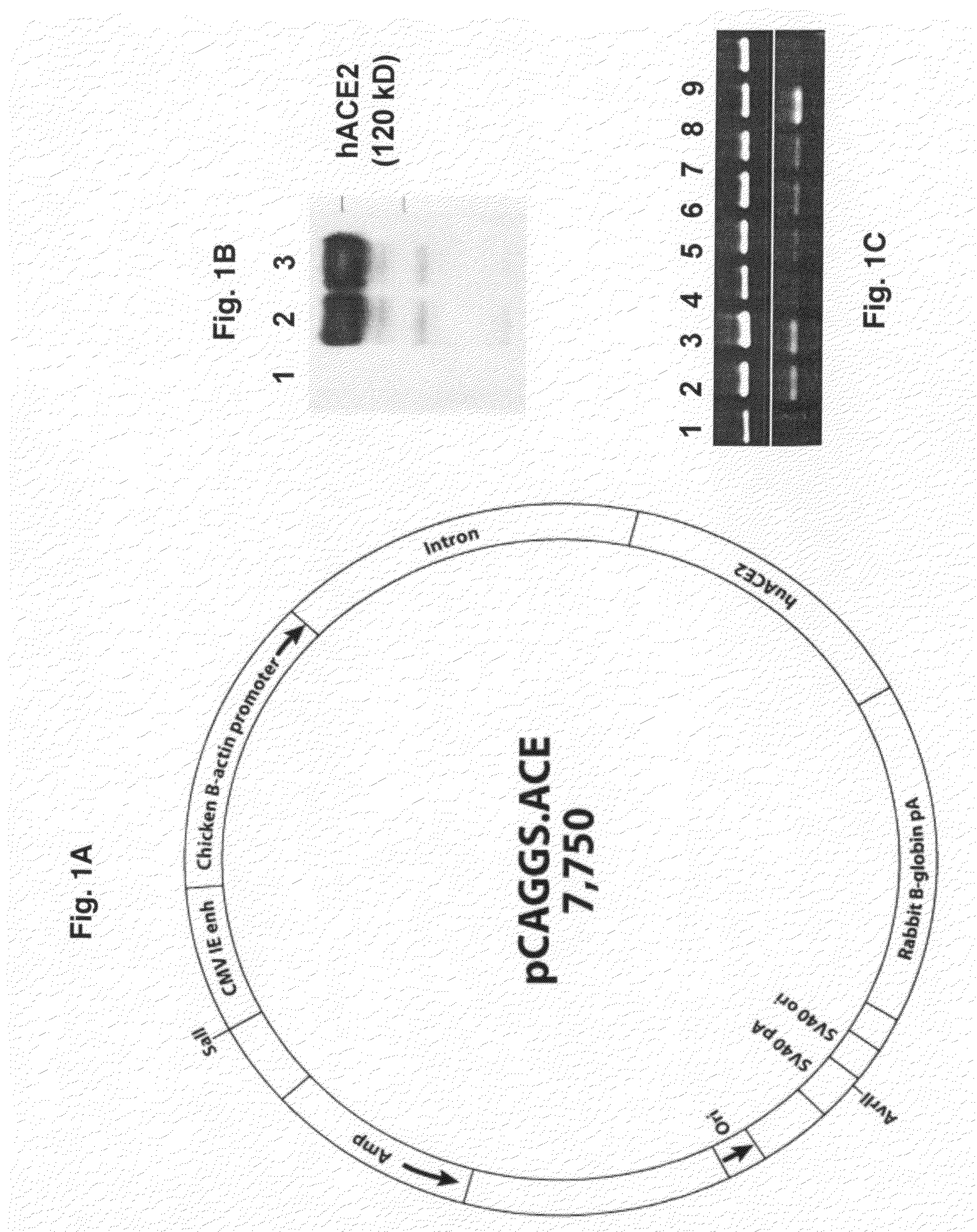
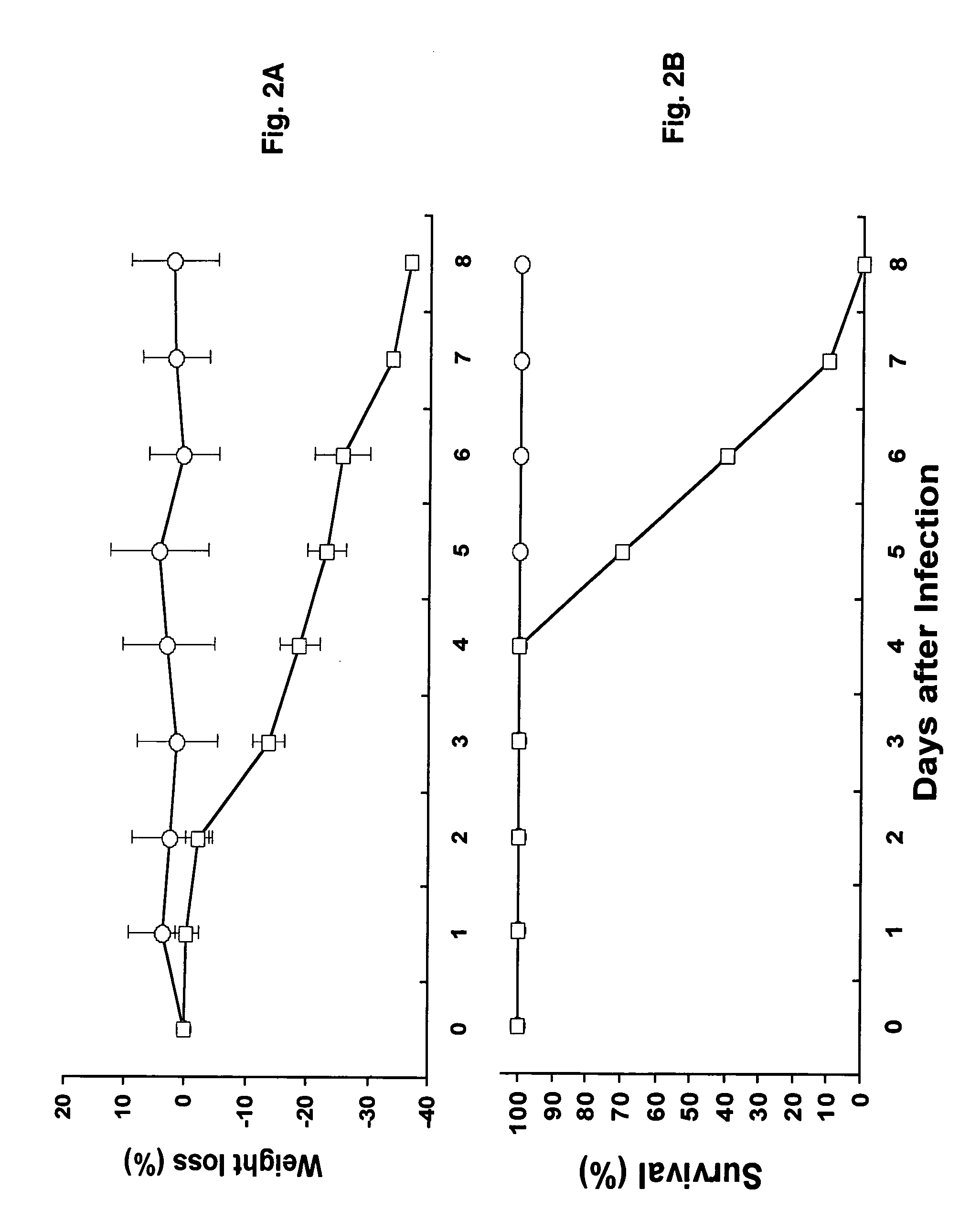
Transgenic Mouse Lines Expressing Human Ace2 and Uses Thereof
InactiveUS20090083865A1Prevents and alleviates symptomShortens courseHydrolasesBiological material analysisClinical manifestationMortality rate
Animal models for severe acute respiratory syndrome-coronavirus infection of humans are needed to elucidate SARS pathogenesis and develop vaccines and antivirals. Transgenic mice were developed expressing human angiotensin-converting enzyme 2, a functional receptor for the virus, under the regulation of a global promoter. A transgenic lineage, designated AC70, was among the best characterized against SARS coronavirus infection, showing weight loss and other clinical manifestations before reaching 100% mortality within 8 days after intranasal infection. Inflammatory mediators were also detected in these tissues, coinciding with high levels of virus replication. In contrast, infected transgene-negative mice survived without showing any clinical illness. The severity of the disease developed in these transgenic mice, AC70 in particular, makes these mouse models valuable not only for evaluating the efficacy of antivirals and vaccines, but also for studying SARS coronavirus pathogenesis and infection by other coronaviruses utilizing human ACE2 for viral entry into cells.
Owner:CHAN TEH SHENG +3
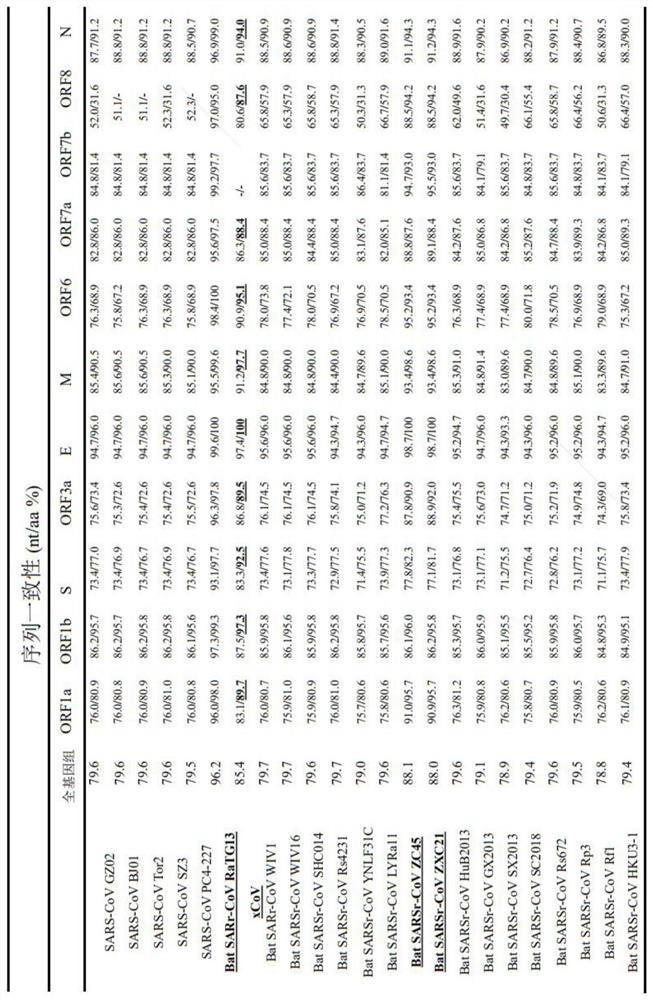
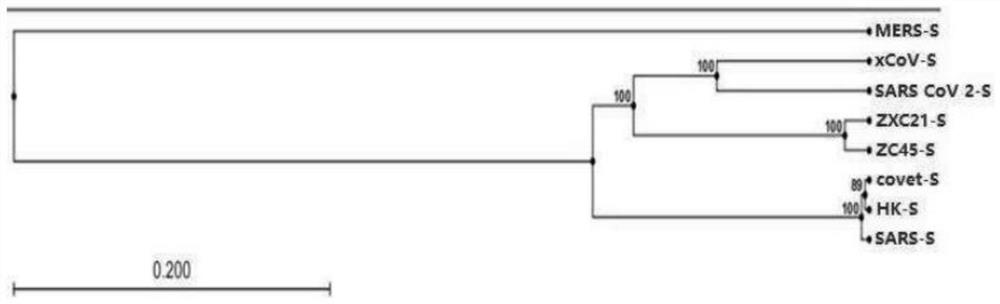
Pangolin coronavirus xCoV and application thereof and application of medicine in resisting coronavirus infection
ActiveCN113046327AHigh homologyProof of InhibitionSsRNA viruses positive-senseMicrobiological testing/measurementReceptorCepharanthine
The invention provides a coronavirus separated from pangolin. The coronavirus is named as pangolin coronavirus xCoV, the homology of the coronavirus xCoV and S protein of SARS-COV-2 reaches 92.5%, receptors of xCoV infected cells and the SARS-COV-2 are consistent and are angiotensin converting enzyme 2 (ACE2). However, the xCoV does not infect people, is very safe to people, can be used for screening active drugs and vaccines for resisting the SARS-COV-2 virus, and can also be used for preparing attenuated vaccines or inactivated vaccines for resisting the SARS-COV-2 virus. Based on the pangolin coronavirus xCoV, a plurality of active compounds with anti-coronavirus activity are obtained by screening, EC50, CC50 and SI evaluation is performed on cepharanthine (stephanine), silamectin and mefloquine hydrochloride (mefloquine) in the active compounds, and the xCoV inhibition mechanism of cepharanthine is analyzed and researched through transcriptome sequencing.
Owner:BEIJING UNIV OF CHEM TECH
Novel coronavirus (COVID-19) neutralizing antibody detection kit and detection method
PendingCN113156131AReduce contact areaIncrease contact areaBiological material analysisBiological testingNeutralising antibodyFluorescence immunoassay analyzer
The invention discloses a novel coronavirus (COVID-19) neutralizing antibody detection kit, which is characterized by comprising: a detection card. The detection card comprises a card shell and a test strip arranged in the card shell, the test strip comprises a sample pad, a fluorescence combination pad, a nitrocellulose membrane, a water absorption pad and a backing plate. The fluorescent combination pad comprises a detection line fluorescent conjugate and a quality control line fluorescent conjugate, the detection line fluorescent conjugate is a novel recombinant coronavirus spinous process glycoprotein (RBD) marked by fluorescent microspheres, and the nitrocellulose membrane is coated with a detection line formed by angiotensin converting enzyme 2 (ACE2). And the nitrocellulose membrane is also coated with a quality control line formed by IgY, IgG or DNP antibodies. The kit is combined with a fluorescence immunoassay analyzer to detect a sample, and the kit is high in detection sensitivity, good in reproducibility, high in stability and convenient to operate.
Owner:深圳市爱康试剂有限公司
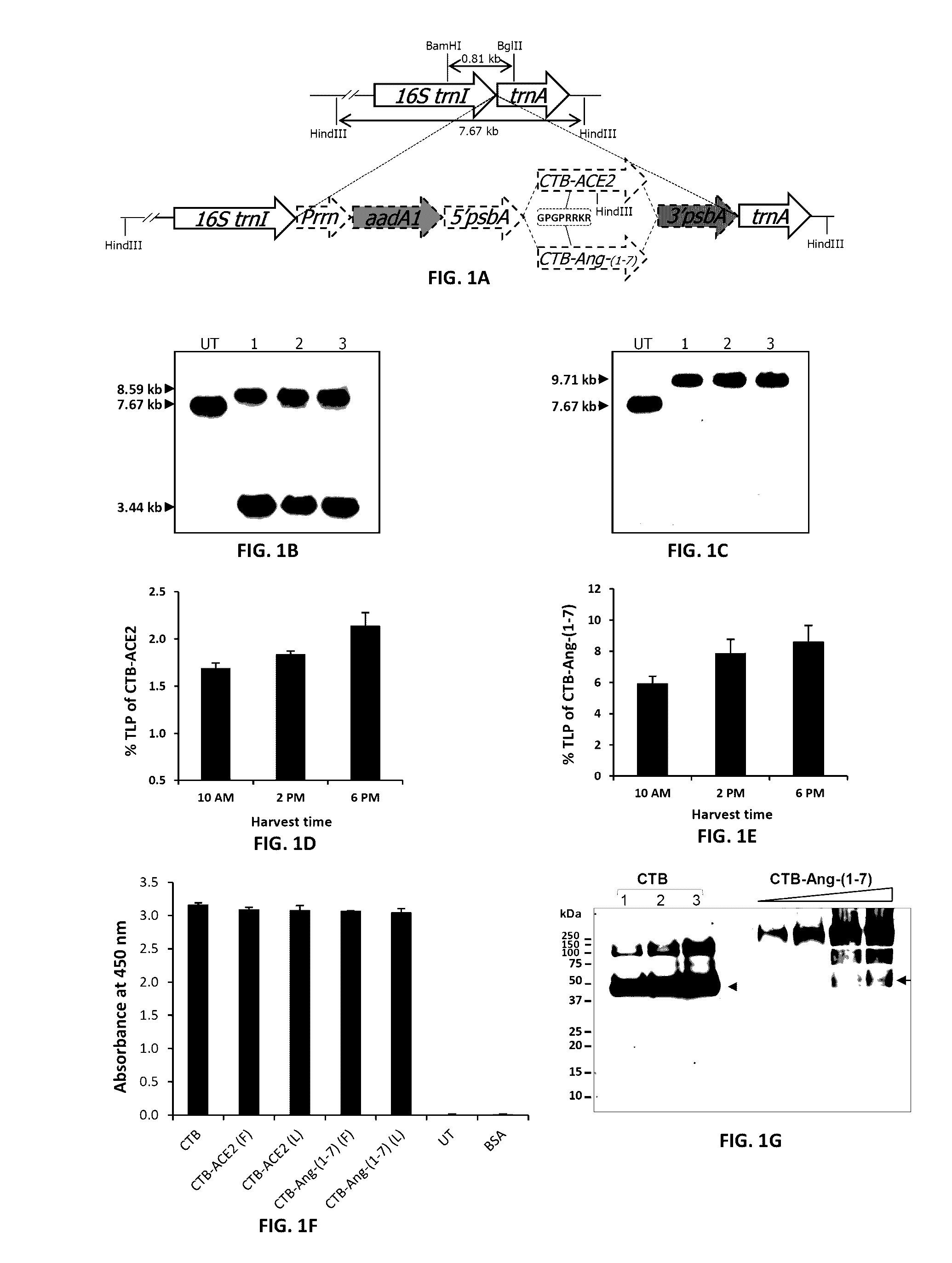
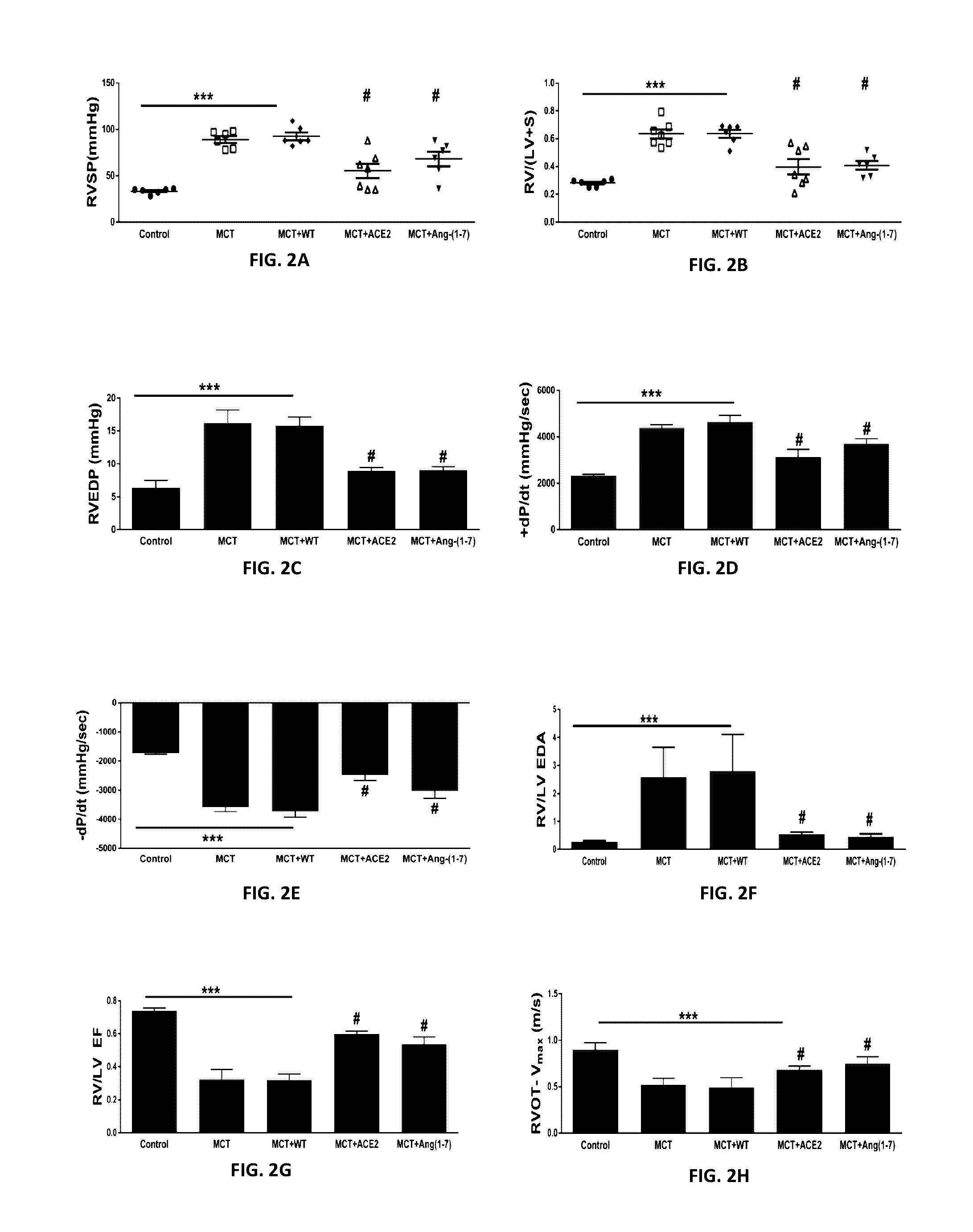
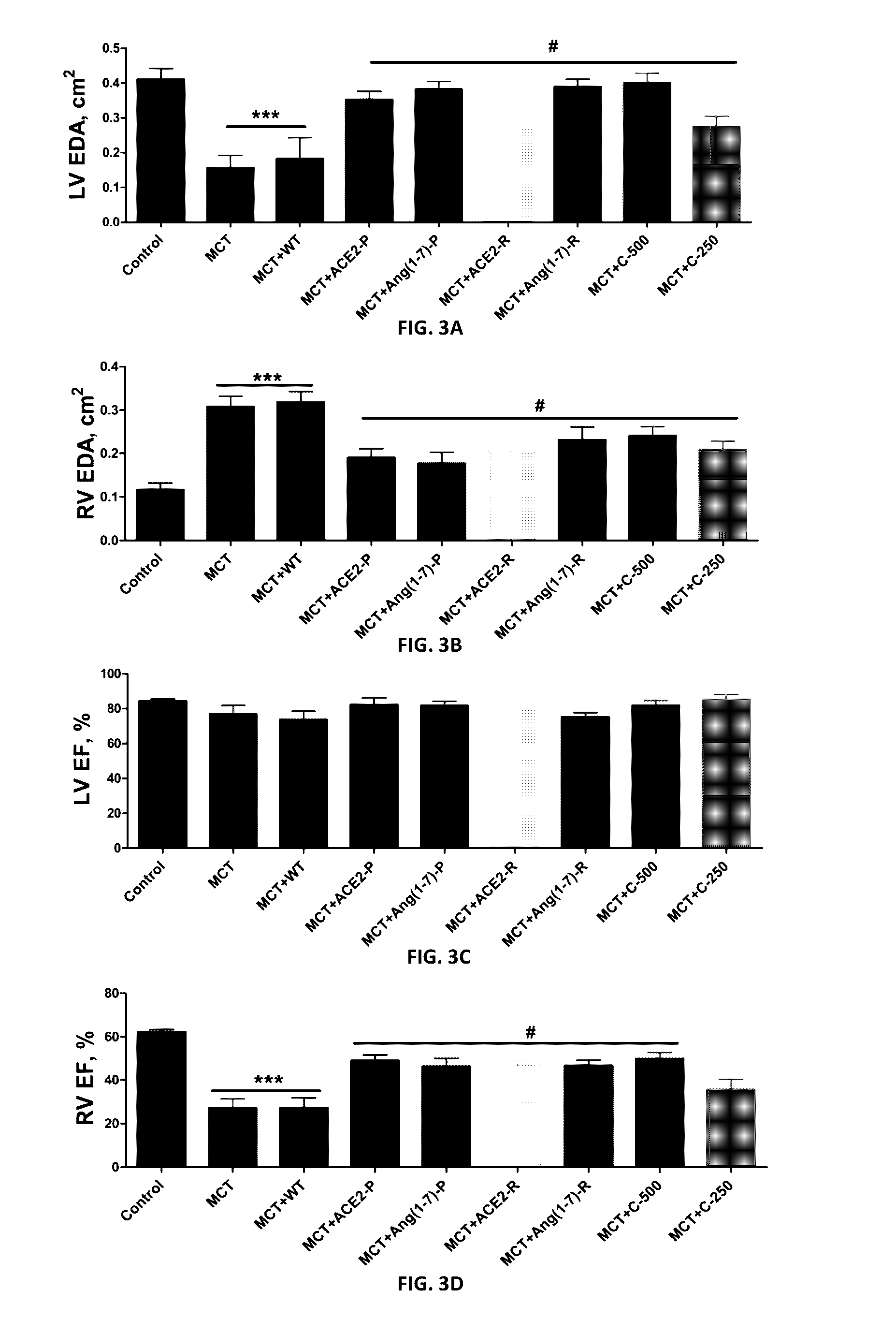
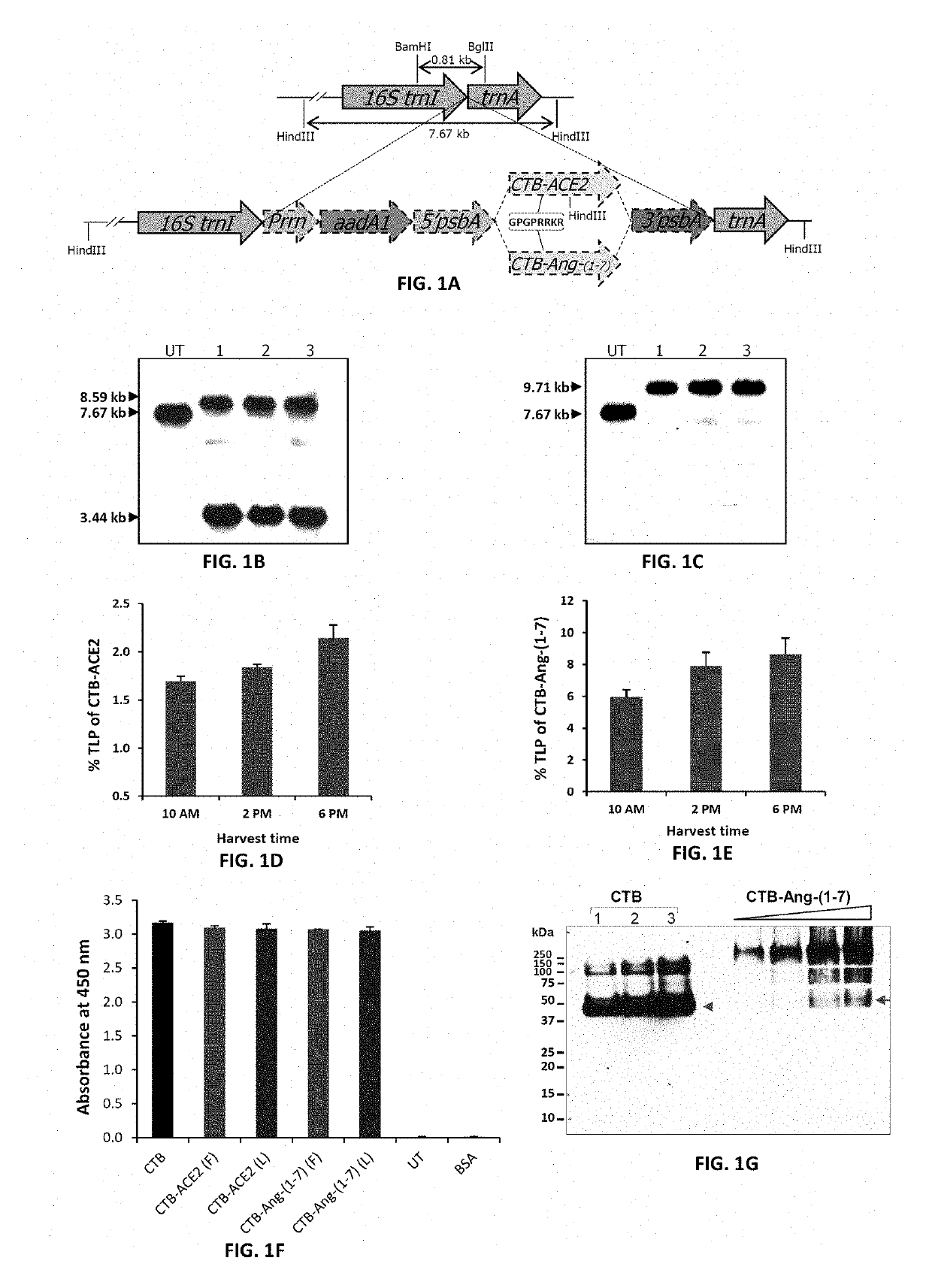
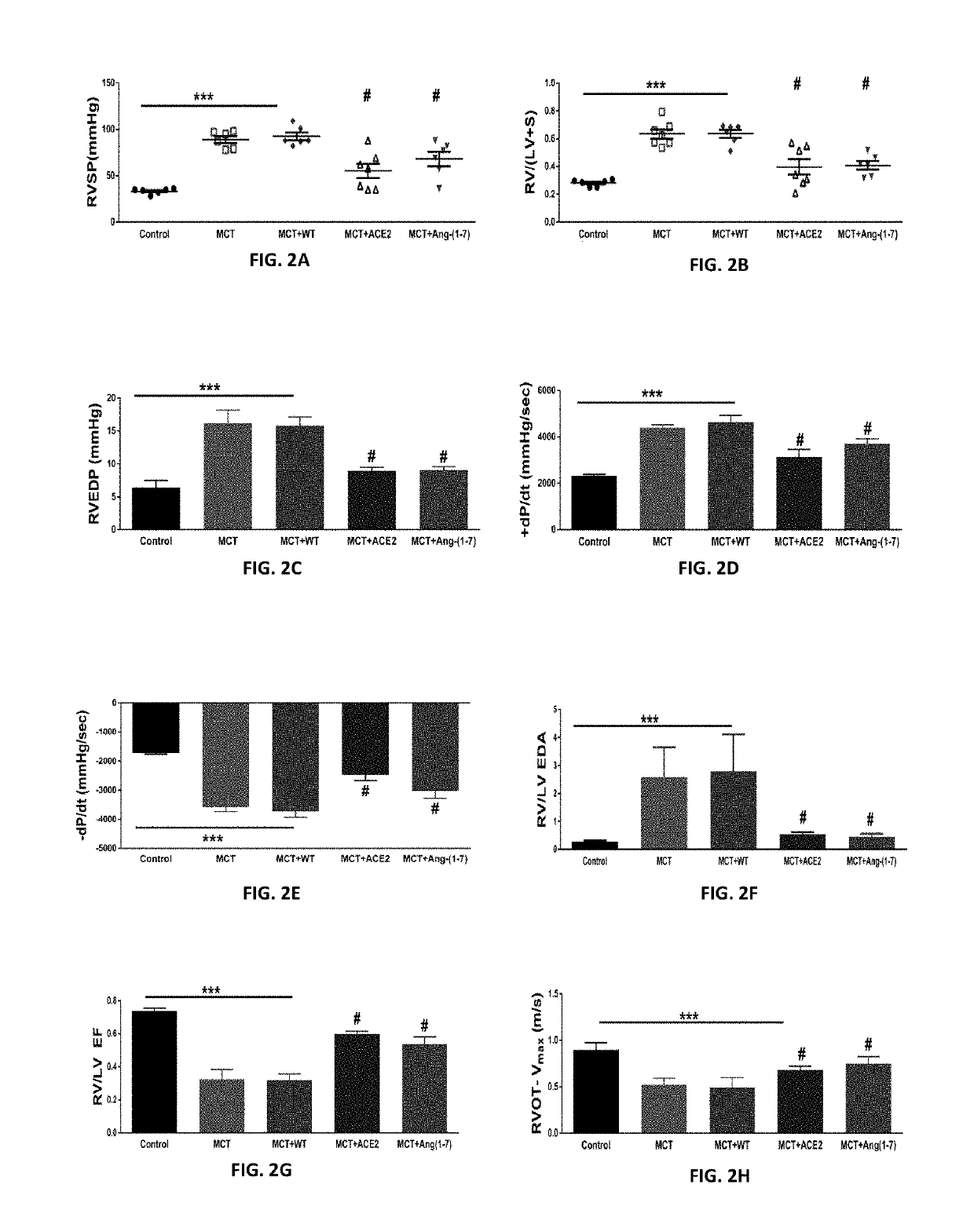
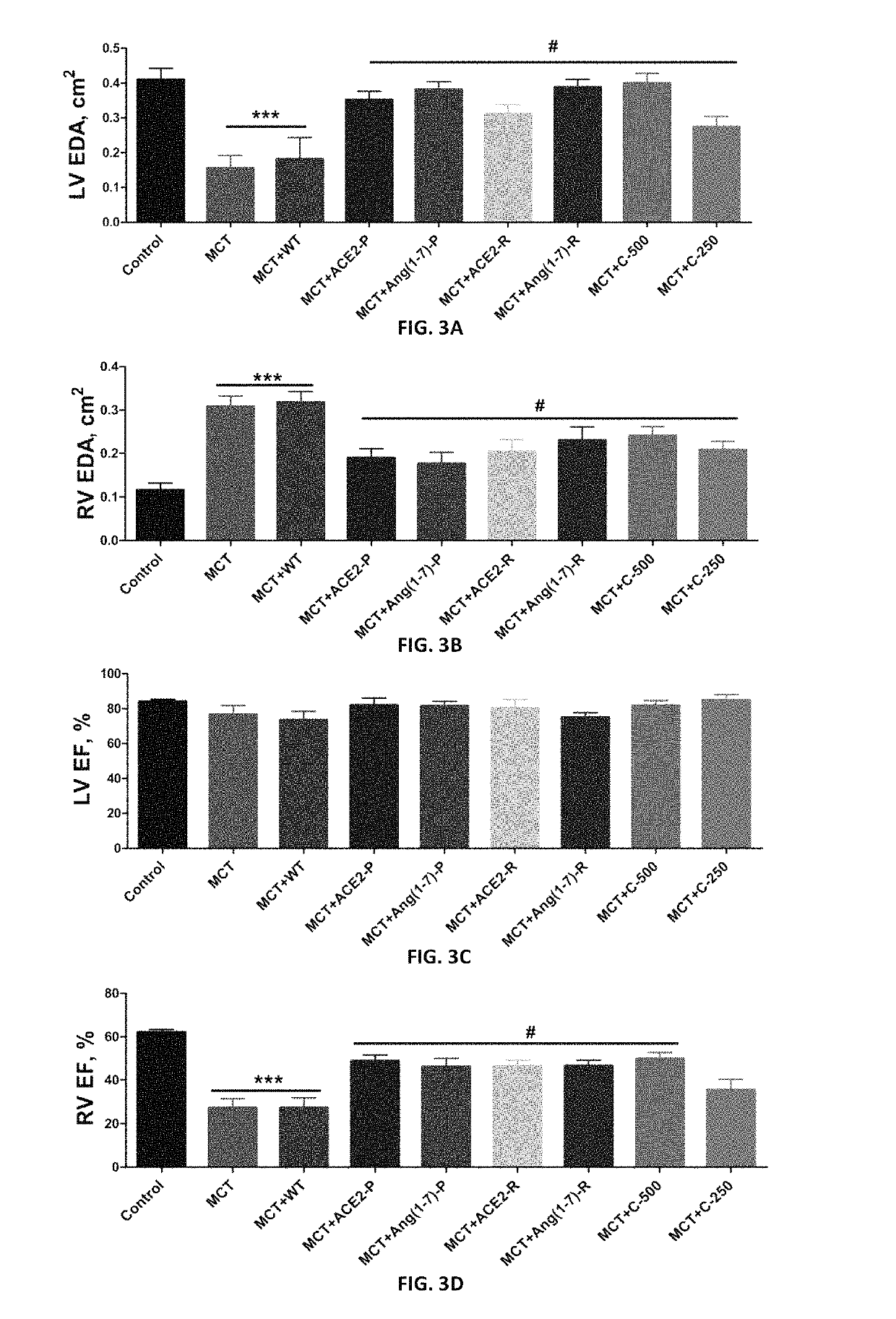
Oral delivery of angiotensin converting enzyme 2 (ACE2) or angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension, cardiac dysfunction and development of autoimmune and experimentally induced ocular disorders
ActiveUS20160331816A1Improved right heart functionDecreased pulmonary vessel wall thicknessPowder deliveryPeptide/protein ingredientsDiseaseUveitis
Emerging evidence indicates that diminished activity of the vasoprotective axis of the renin-angiotensin system, constituting angiotensin converting enzyme2 (ACE2) and its enzymatic product, angiotensin-(1-7) [Ang-(1-7)] contribute to pulmonary hypertension (PH). However, clinical success for long-term delivery of ACE2 or Ang-(1-7) would require stability and ease of administration to increase patient compliance. Chloroplast expression of therapeutic proteins enables their bioencapsulation within plant cells to protect from acids and gastric enzymes; fusion to a transmucosal carrier facilitates effective systemic absorption. Oral feeding of rats with bioencapsulated ACE2 or Ang-(1-7) attenuated monocrotaline (MCT)-induced increase in right ventricular systolic pressure, decreased pulmonary vessel wall thickness and improved right heart function in both prevention and reversal protocols. Furthermore, combination of ACE2 and Ang-(1-7) augmented the beneficial effects against cardio-pulmonary pathophysiology induced by MCT administration.Experiments have also been performed which indicate that this approach is also suitable for the treatment or inhibition of experimental uveitis and autoimmune uveoretinitis These studies provide proof-of-concept for a novel low-cost oral ACE2 or Ang-(1-7) delivery system using transplastomic technology for pulmonary and ocular disease therapeutics.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Angiotensin converting enzyme homolog and uses therefor
InactiveUS6884771B1Good determineEffectively prescribeAngiotensinsPeptide/protein ingredientsAngiotensin-converting enzymeAllergic condition
The present invention relates to the discovery of novel genes encoding an angiotensin converting enzyme, Angiotensin Converting Enzyme-2 (ACE-2). The invention provides therapeutics, prognostic and diagnostics methods for treating blood pressure related disorders as well as various types of allergic conditions, among others. Also disclosed are screening assays for identifying compounds for treating and preventing these conditions.
Owner:MILLENNIUM PHARMA INC
Oral delivery of angiotensin converting enzyme 2 (ACE2) or angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension, cardiac dysfunction and development of autoimmune and experimental induced ocular disorders
ActiveUS10314893B2Good effectAvoid poor resultsPowder deliveryPeptide/protein ingredientsDiseaseUveitis
Emerging evidence indicates that diminished activity of the vasoprotective axis of the renin-angiotensin system, constituting angiotensin converting enzyme2 (ACE2) and its enzymatic product, angiotensin-(1-7) [Ang-(1-7)] contribute to pulmonary hypertension (PH). However, clinical success for long-term delivery of ACE2 or Ang-(1-7) would require stability and ease of administration to increase patient compliance. Chloroplast expression of therapeutic proteins enables their bioencapsulation within plant cells to protect from acids and gastric enzymes; fusion to a transmucosal carrier facilitates effective systemic absorption. Oral feeding of rats with bioencapsulated ACE2 or Ang-(1-7) attenuated monocrotaline (MCT)-induced increase in right ventricular systolic pressure, decreased pulmonary vessel wall thickness and improved right heart function in both prevention and reversal protocols. Furthermore, combination of ACE2 and Ang-(1-7) augmented the beneficial effects against cardio-pulmonary pathophysiology induced by MCT administration.Experiments have also been performed which indicate that this approach is also suitable for the treatment or inhibition of experimental uveitis and autoimmune uveoretinitis These studies provide proof-of-concept for a novel low-cost oral ACE2 or Ang-(1-7) delivery system using transplastomic technology for pulmonary and ocular disease therapeutics.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
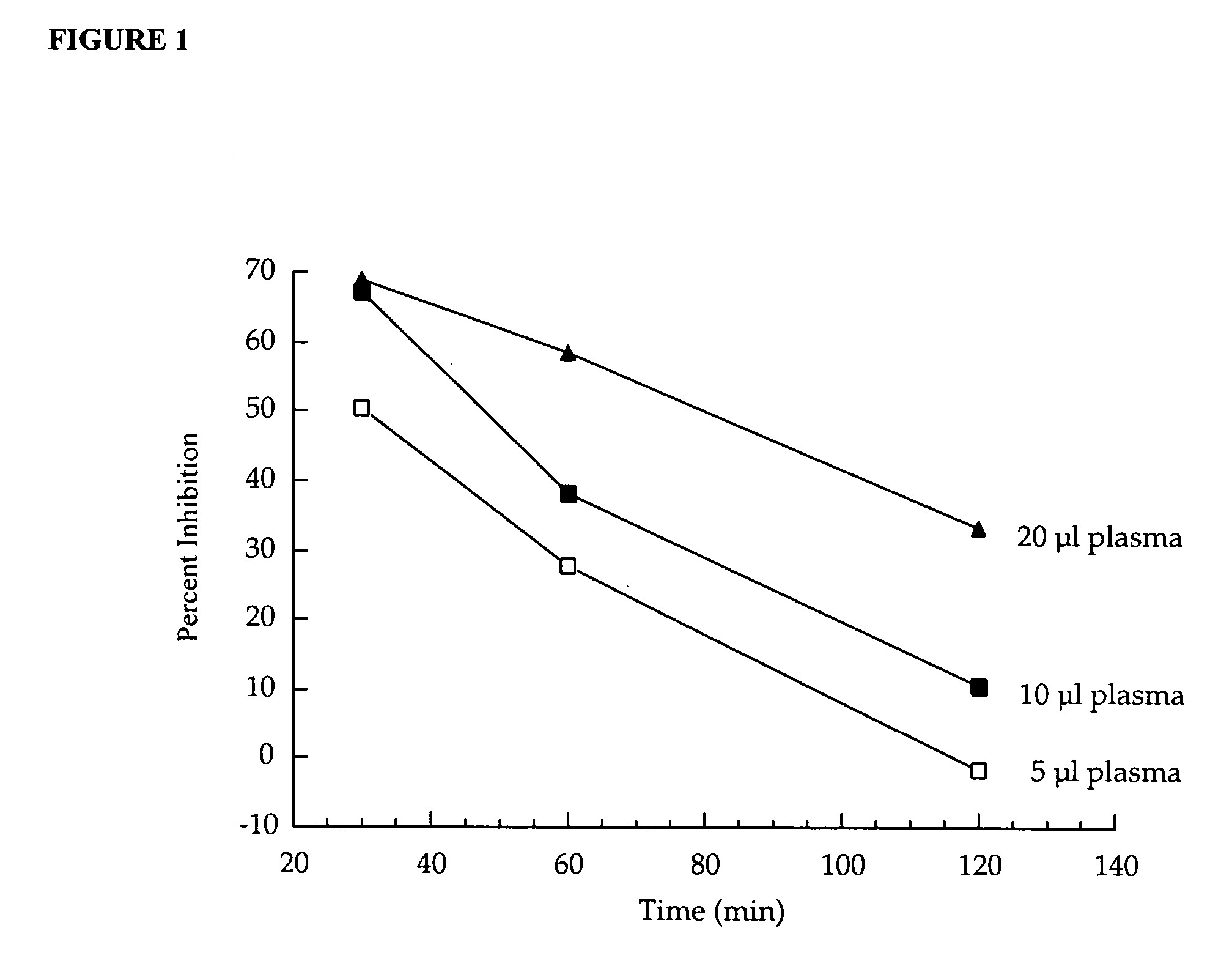
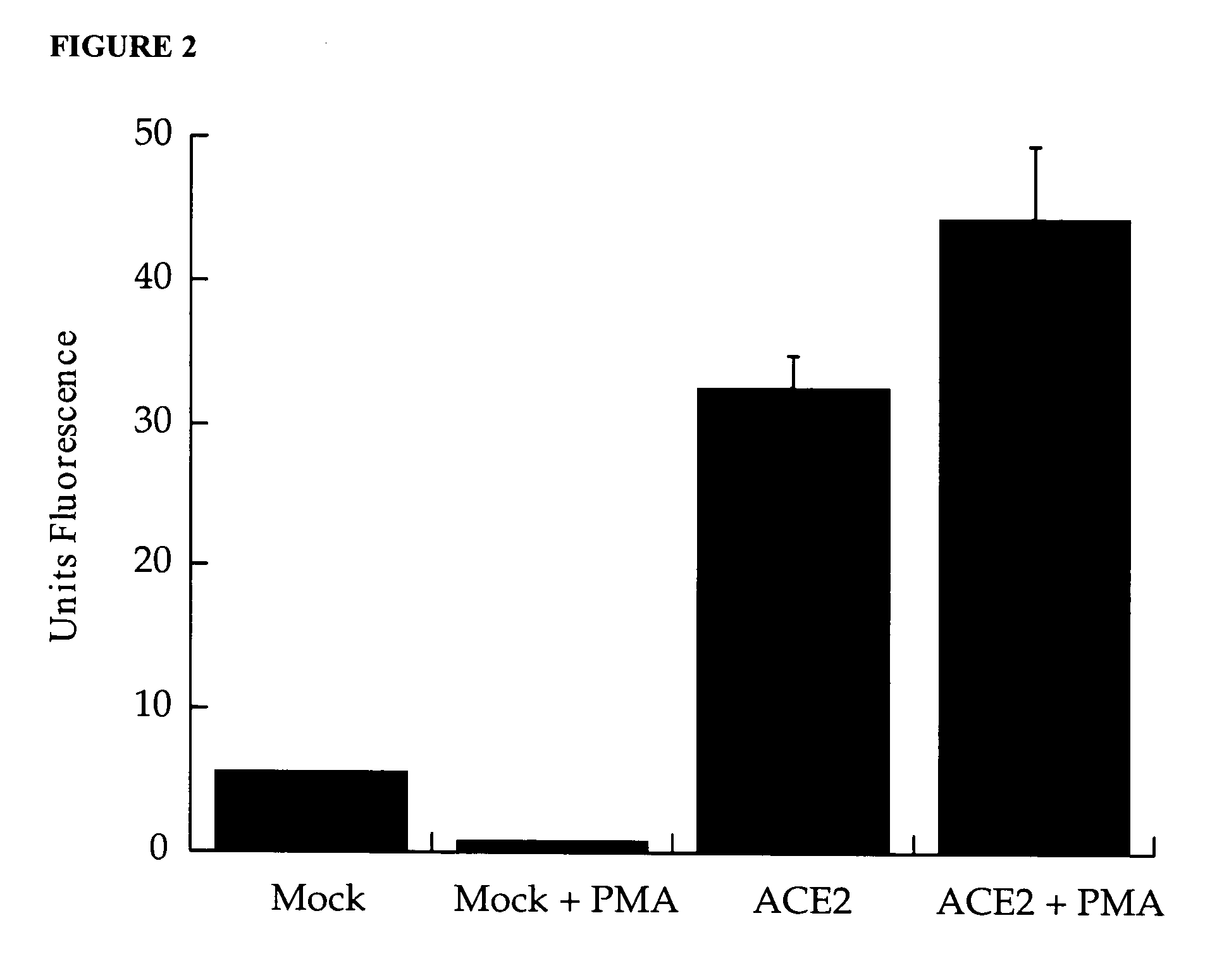
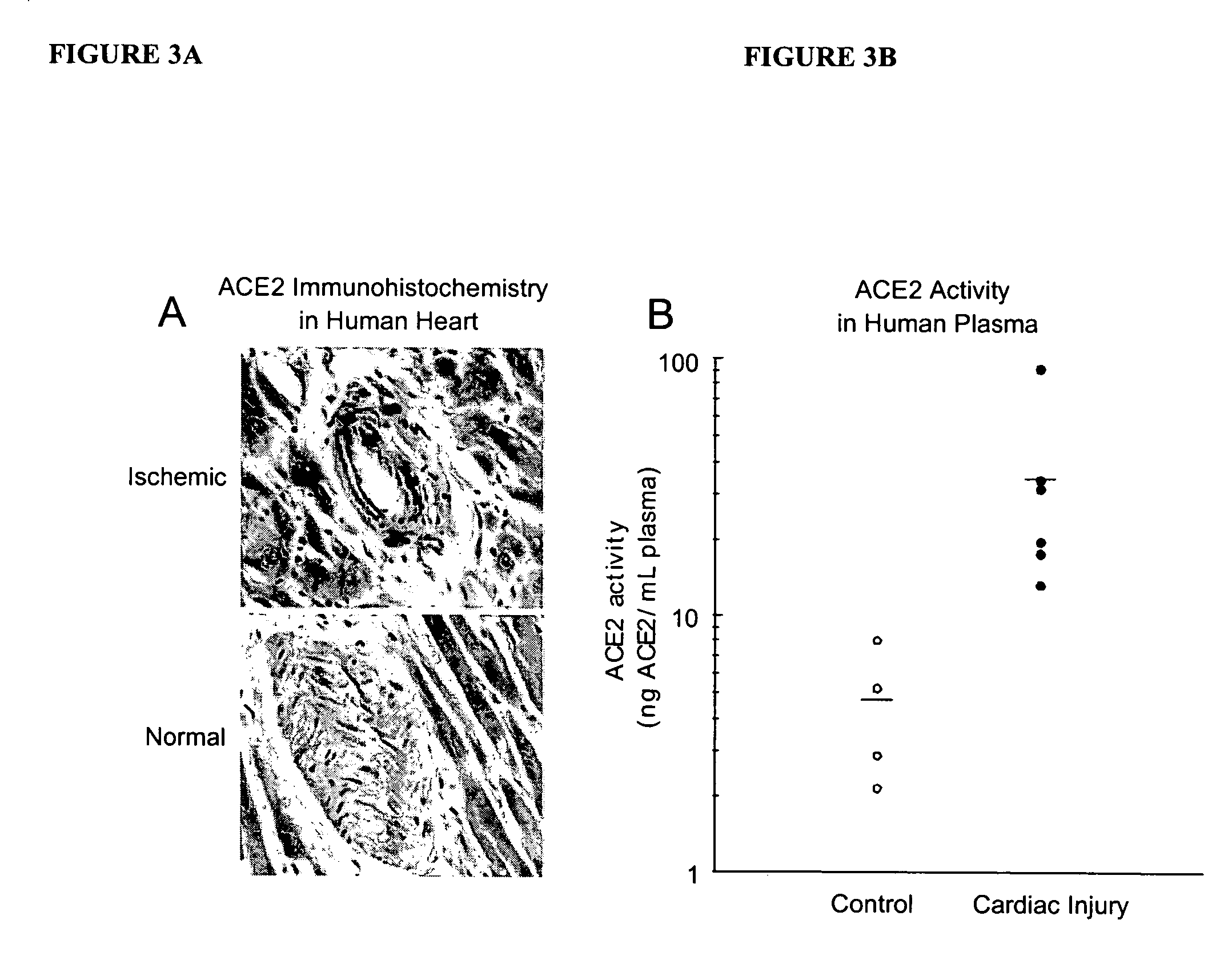
Detection of plasma ACE2
InactiveUS20070042452A1Microbiological testing/measurementDisease diagnosisPlasma biomarkersIschaemic heart disease
A method of assessing a subject for aberrant cardiac function is described. The method particularly involves the measurement of a plasma biomarker, namely metallopeptidase angiotensin-converting enzyme 2 (ACE2), implicated in ischaemic heart disease.
Owner:MONASH UNIV +1
Magnetic beads for detection of COVID-19 and preparation method of magnetic beads
InactiveCN111411104AImprove detection efficiencyImprove accuracyBiological material analysisOn/in organic carrierAngiotensin-converting enzymePolyol
The invention discloses magnetic beads for detection of COVID-19 and a preparation method of the magnetic beads. The preparation method comprises the steps of firstly, synthesizing dextran-modified Fe3O4 nano magnetic beads by adopting a high-temperature polyol method, and then preparing Fe3O4 / SiO2 / PMO composite nano magnetic beads with a yolk-shell structure by adopting a simple and controllablegrowth-induced corrosion method; and coupling angiotensin converting enzyme 2 (ACE2) to the dextran-modified Fe3O4 / SiO2 / PMO nano magnetic beads. The ACE2 is specifically bound with novel coronavirus in an adsorption process by utilizing the specificity of the ACE2 to novel coronavirus, thereby quickly separating and enriching novel coronavirus from a complex sample with extremely low novel coronavirus content to improve the detection efficiency and positive rate of the COVID-19. The magnetic beads have the advantages of high accuracy, high sensitivity, simple and convenient operation and strong specificity, thereby providing an effective way for early screening of the COVID-19.
Owner:李长桂
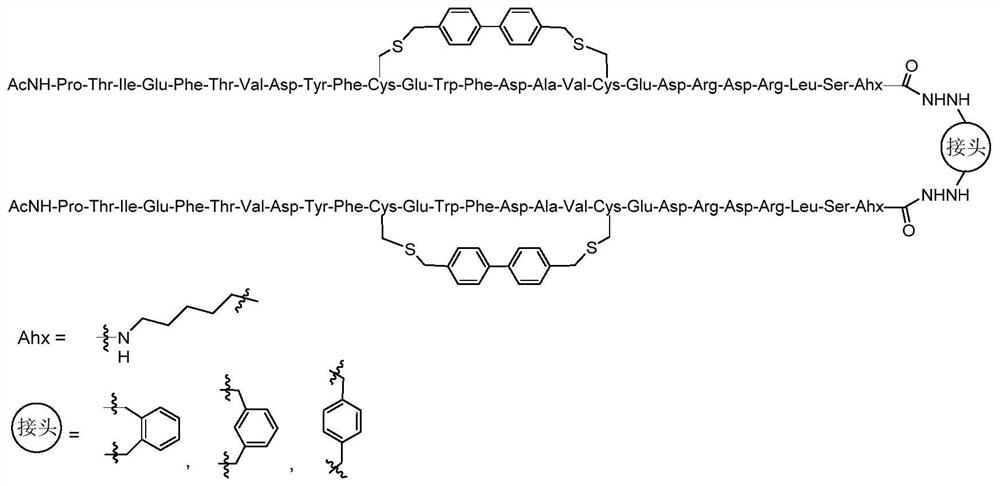
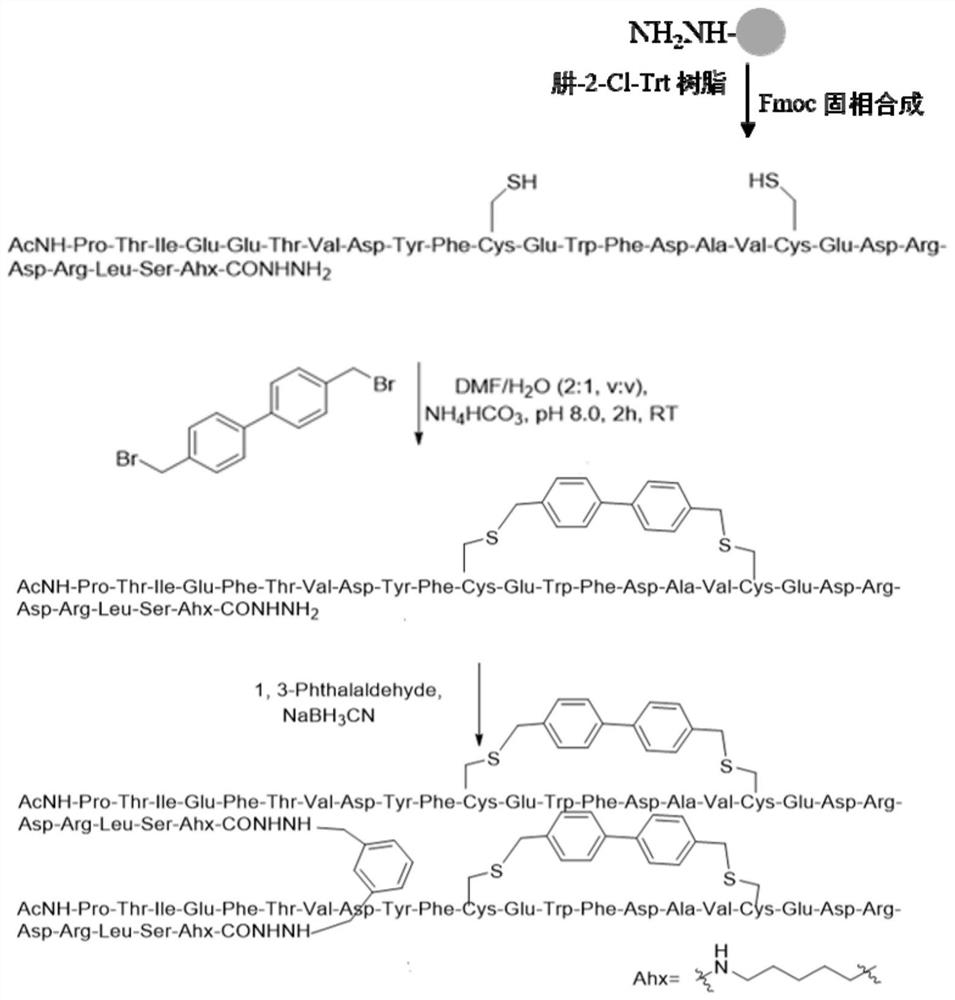
Cyclic peptide combined with RBD site of novel coronavirus as well as preparation method and application of cyclic peptide
ActiveCN113278054APeptide/protein ingredientsBiological material analysisCyclic peptideAngiotensin-converting enzyme
The invention discloses a cyclic peptide combined with an RBD site of novel coronavirus as well as a preparation method and application of the cyclic peptide, and particularly relates to a cyclic polypeptide molecule obtained through in-vitro amino acid side chain cyclization, amino acid point mutation and dimerization modification on the basis of an angiotensin converting enzyme 2 (ACE2) core sequence combined with the RBD of novel coronavirus. The cyclic polypeptide disclosed by the invention can be used for preparing a polypeptide drug with an SARS-CoV-2 virus inhibition effect, or used for preparing a detection reagent for detecting SARS-CoV-2, and a bioactive lead molecule with commercial value is provided for treatment and detection of SARS-CoV-2.
Owner:ANHUI UNIVERSITY
Small- molecule inhibitor for blocking combination of COVID-19 spinous protein and human angiotensin converting enzyme 2 and application thereof
ActiveCN112457281AOrganic chemistryPeptide/protein ingredientsPharmaceutical drugAngiotensin-converting enzyme 2
The invention belongs to the technical field of biology and medicine, and relates to a small-molecule inhibitor for blocking combination of COVID-19 spinous protein and human angiotensin converting enzyme 2 and application of the small-molecule inhibitor. The small molecular inhibitor which has an inhibiting effect on the combination of the COVID-19 spinous protein and the human angiotensin converting enzyme 2 and blocks the COVID-19 transferred into cells within the range of 0.5-500 mu M is found through virtual screening, experiments of blocking the combination of the COVID-19 spinous protein and the human angiotensin converting enzyme 2 and antiviral transcription experiments, The small-molecule inhibitor can be used for preparing medicines for preventing and treating novel coronavirusinfection.
Owner:DALIAN UNIV OF TECH +1
Novel coronavirus neutralizing antibody detection test paper
PendingCN112798797ALower titerSimple detection operationBiological material analysisBiological testingViral antibodyCoronavirus antibody
The invention relates to the field of biomedical detection and particularly relates to novel coronavirus neutralizing antibody detection test paper, a kit, a detection method and application. The novel coronavirus neutralizing antibody detection test paper comprises a substrate, and a sample loading pad, a colloidal gold adsorption pad, an antibody bearing film and a water absorption pad which are overlapped on the substrate in sequence; wherein a detection line T1, a detection line T2 and a quality control line C are arranged on the antibody bearing film at intervals, the detection line T2 is close to the colloidal gold adsorption pad, and the quality control line C is close to the water absorption pad; the colloidal gold absorption pad is coated with a colloidal gold labeled novel coronavirus S-RBD antigen and a colloidal gold labeled antibody irrelevant to a new coronavirus, and the detection line T1 is coated with a second antibody of a new coronavirus antibody IgG; the detection line T2 is coated with angiotensin converting enzyme 2; the quality control line C is coated with a secondary antibody which is specifically combined with an antibody irrelevant to the colloidal gold labeled new coronavirus; the test paper can be used for detecting the novel coronavirus neutralizing antibody, and is simple to operate, short in time, and high in specificity and sensitivity during detection.
Owner:NANTONG EGENS BIOTECH
High-affinity human angiotensin converting enzyme 2 (ACE2) mutant and application thereof
PendingCN114107263AEffective therapeuticEffective preventionPeptide/protein ingredientsAntibody mimetics/scaffoldsMutantBlood vessel
The invention belongs to the technical field of bioengineering, and discloses a high-affinity human angiotensin converting enzyme 2 (ACE2) mutant and application thereof. The high affinity ACE2 mutant or ACE2-Fc protein comprises, relative to the wild type ACE2 protein, one or more mutations selected from the group consisting of sites 25, 27, 31, 34, 79, 82, 324, 326, 330, and 387. The mutant can be effectively combined with SARS-CoV-2 virus S protein, has high affinity, can be effectively used for treatment and prevention of viruses, especially novel coronavirus, and has a wide application prospect.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
Human angiotensin converting enzyme 2-based affinity polypeptide for severe acute respiratory syndrome coronavirus 2
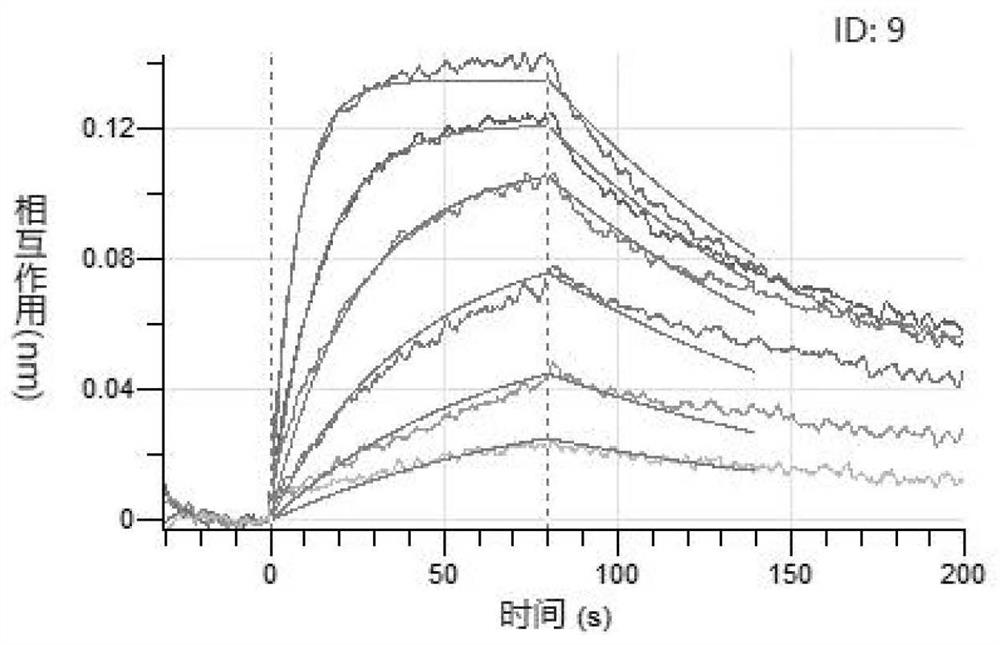
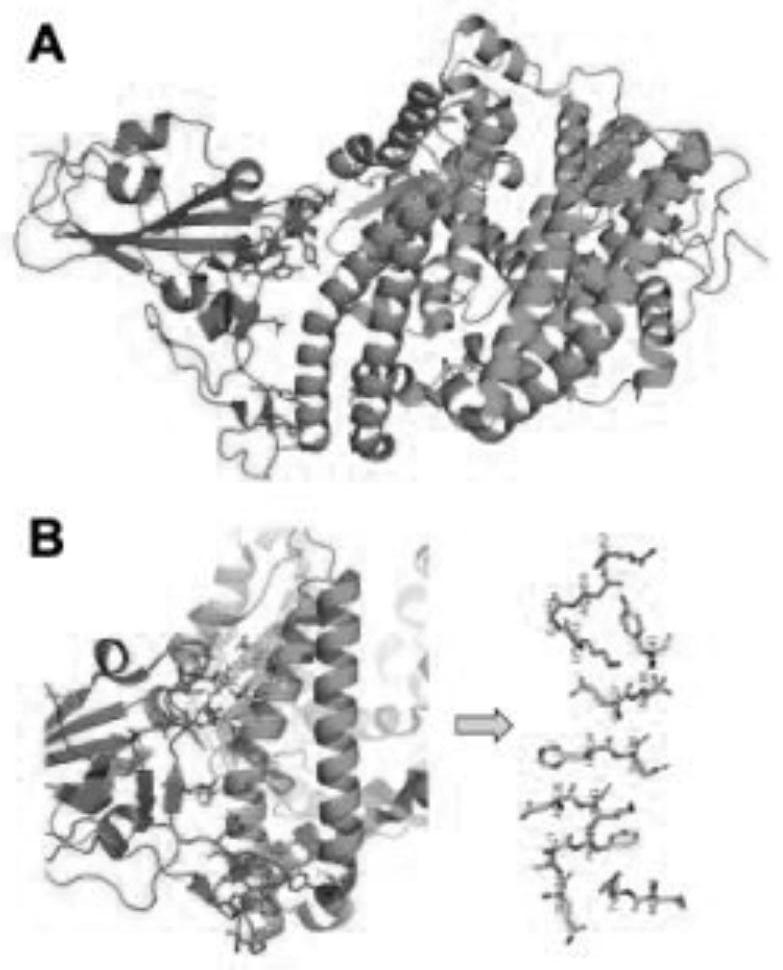
ActiveCN112375754AAvoid interactionNo effectBiological material analysisPeptide preparation methodsBinding siteVirus Binding
The invention provides a human angiotensin converting enzyme 2-based affinity polypeptide for the severe acute respiratory syndrome coronavirus 2, and relates to the technical field of biological materials. Key human angiotensin converting enzyme 2 amino acid residues of binding sites of human angiotensin converting enzyme 2 and the severe acute respiratory syndrome coronavirus 2 are extracted toreconstruct a peptide library, and the human angiotensin converting enzyme 2-based affinity polypeptide for the severe acute respiratory syndrome coronavirus 2 is obtained by screening; and then, a biological detection reagent is prepared from the affinity polypeptide, and thus, a new method is provided for detection of the severe acute respiratory syndrome coronavirus 2.
Owner:TSINGHUA UNIV
ACE2- and TMPRSS2-Targeted Compositions and Methods for Treating COVID-19
PendingUS20220098283A1Reduce the possibilityInhibit bindingVectorsInorganic non-active ingredientsHuman cellAngiotensin-converting enzyme 2
This invention provides a composition comprising (a) a first monoclonal antibody that (i) specifically binds to the extracellular portion of human angiotensin converting enzyme 2 (hACE2), (ii) specifically inhibits binding of SARS-CoV-2 to the extracellular portion of hACE2, and (iii) does not significantly inhibit the ability of hACE2 to cleave angiotensin II and / or a synthetic MCA-based peptide; and (b) a second monoclonal antibody that (i) specifically binds to the extracellular portion of human TMPRSS2 (hTMPRSS2), and (ii) specifically inhibits the entry into hACE2+ / hTMPRSS2+ human cells of a pseudovirus bearing SARS-CoV-2 S protein. This invention also provides related recombinant AAV vectors, recombinant AAV particles, compositions, prophylactic and therapeutic methods, and kits.
Owner:MADDON ADVISORS LLC
Nanoparticle-rhACE-2 compound for blocking coronavirus from infecting target cells, preparation method and application of nanoparticle-rhACE-2 compound
InactiveCN113599536AHigh mutation rateAvoid infectionPowder deliveryPeptide/protein ingredientsViral ReceptorNanoparticle
The invention discloses a nanoparticle-rhACE-2 compound for blocking coronavirus from infecting target cells, a preparation method and application of the nanoparticle-rhACE-2 compound. The preparation method comprises the following steps: S1, preparing and purifying rhACE-2 protein; S2, labelling the rhACE-2 protein with biotin; and S3, preparing the nanoparticle-rhACE-2 compound: washing nanoparticles with PBS for three times, specifically, washing the nanoparticles with 1ml of PBS for the first time, washing the nanoparticles with PBS with the same amount as a sample for the later two times, adding the soluble rhACE-2 protein in the S2 according to different molar ratios, performing incubating for 30 minutes at 37 DEG C to enable the rhACE-2 to be effectively combined to the surfaces of the nanoparticles, and then performing washing with PBS for three times, wherein the product at the moment is the nanoparticle-rhACE-2 compound. According to the invention, a coronavirus receptor angiotensin converting enzyme 2 is used as an antagonist; and the nanoparticles are used as a carrier to block and neutralize virus particles, so that the infection of viruses to target cells is inhibited.
Owner:南京纳科生物材料有限公司
Angiotensin converting enzyme 2 inhibitor, application thereof and anti-coronavirus infection medicine
The invention belongs to the technical field of biological medicine, and particularly relates to an angiotensin converting enzyme 2 inhibitor and application thereof and an anti-coronavirus infection. The angiotensin converting enzyme 2 inhibitor has the chemical structure shown in the general formula (I), R1 and R2 can be selected from hydrogen, halogen, amino, amido, hydroxyl, cyano, alkyl, naphthenic base and alkoxy, X is selected from hetero atoms such as N, O and S, and Y is selected from alkyl or acylamino. Through detection, the angiotensin converting enzyme 2 inhibitor has ACE2 inhibitory activity, can be used as a novel angiotensin converting enzyme 2 inhibitor, and has the potential of being applied to preparation of angiotensin converting enzyme 2 inhibitory drugs, especially anti-coronavirus infection drugs. (I).
Owner:SHENZHEN INNOVATION CENT OF SMALL MOLECULE DRUG DISCOVERY CO LTD
Medicine for preventing or/and treating coronavirus infection and preparation method thereof
ActiveCN113101310AReduced spike proteinReduce the binding forceAntiviralsPteridophyta/filicophyta medical ingredientsBiotechnologyMedicinal herbs
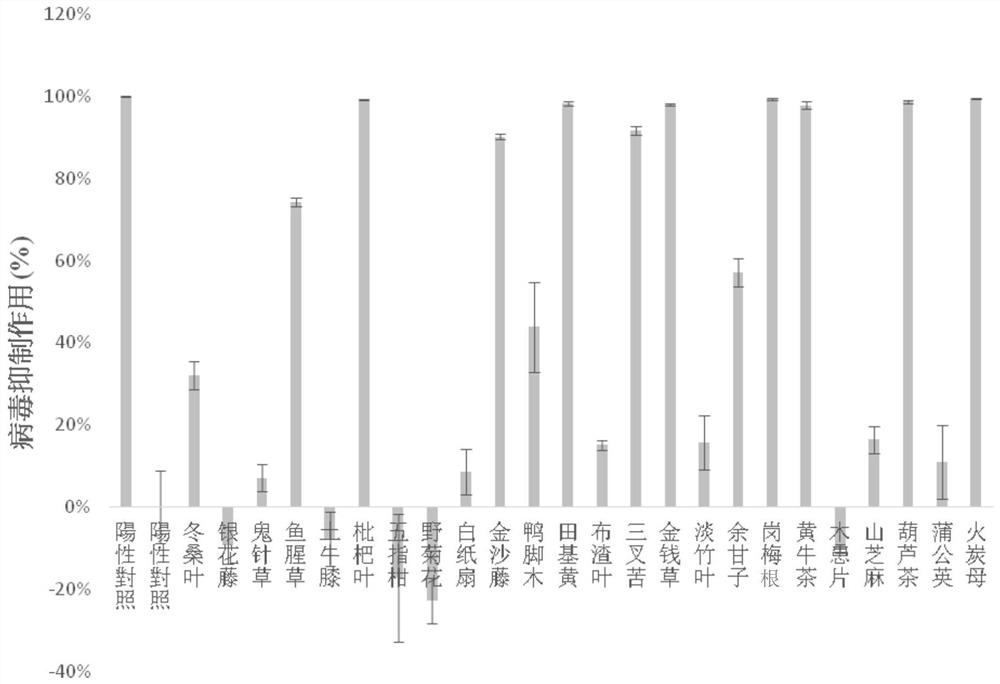
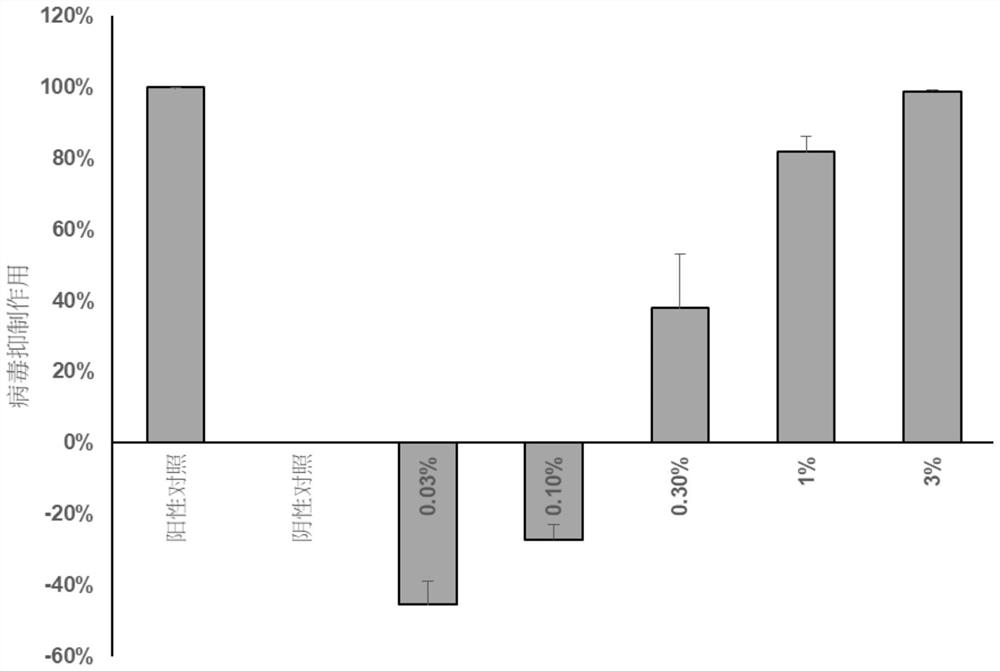
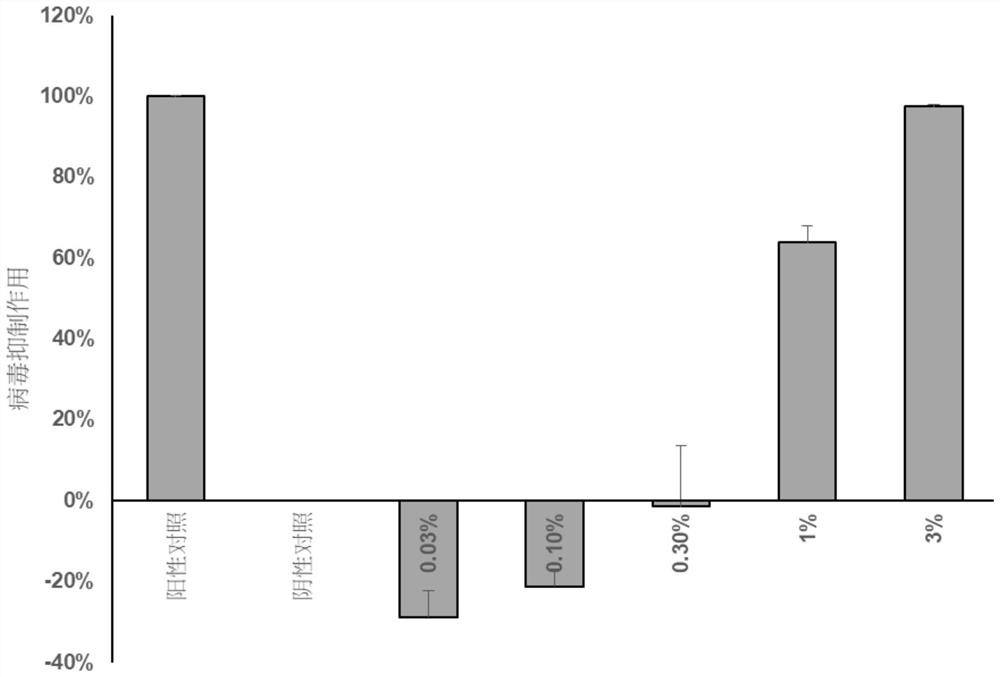
The invention relates to a medicine for preventing or / and treating coronavirus infection and a preparation method thereof. In the traditional Chinese medicine research process, the inventor finds that common Lingnan medicinal materials including Chinese knotweed herb, emblic leafflower fruit, evodia lepta, roughhaired holly root, lysimachia christinae hance, lygodium japonicum, cratoxylum cochinchinense, desmodium triquetrum, loquat leaf and hypericum japonicum can reduce spike protein of the coronavirus and reduce combination of spike protein and angiotensin converting enzyme 2 so that the effect of inhibiting the coronavirus can be achieved. Based on the discovery, the medicinal materials and the traditional Chinese medicine composition containing the medicinal materials are used for preparing the medicine for preventing or / and treating the coronavirus infection, and a new solution is provided for controlling the coronavirus infection.
Owner:THE HONG KONG UNIV OF SCI & TECH
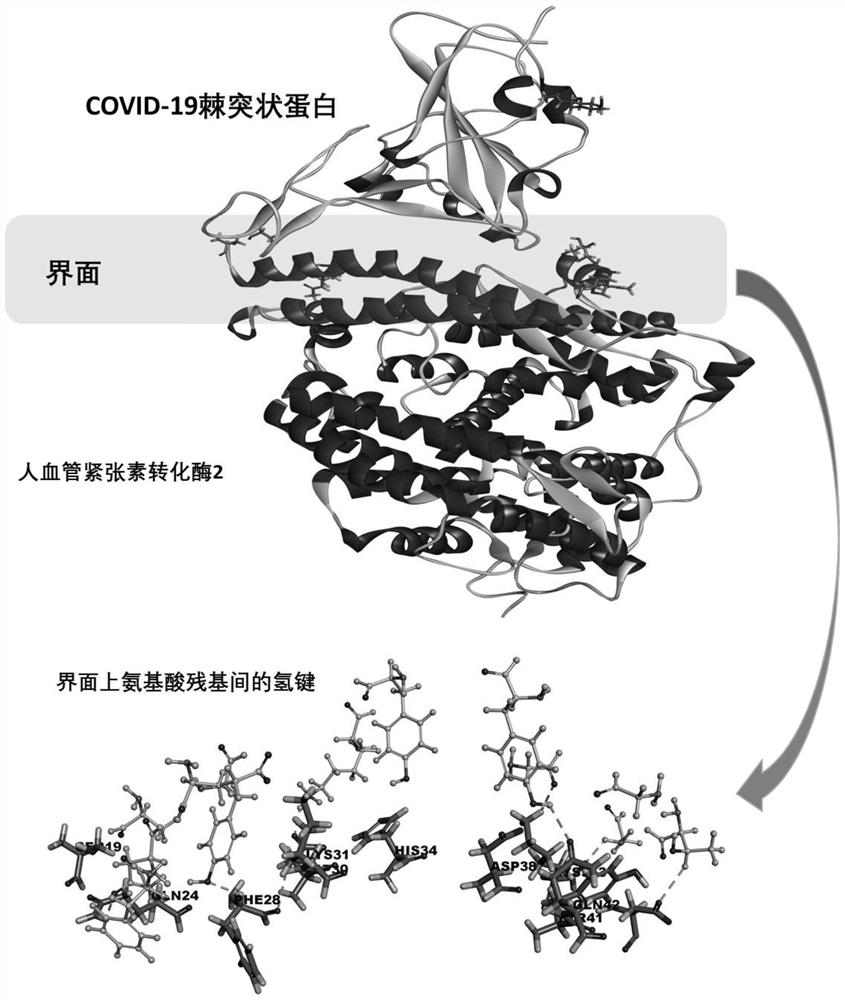
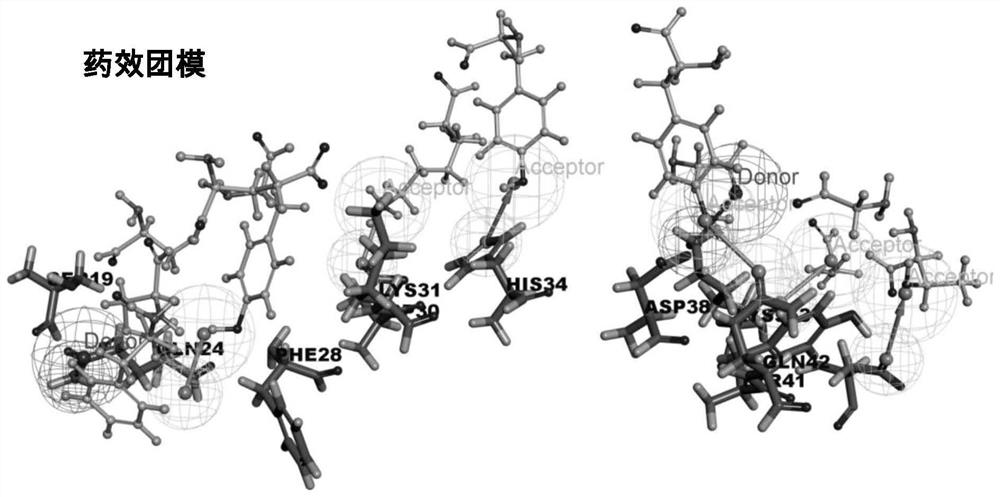
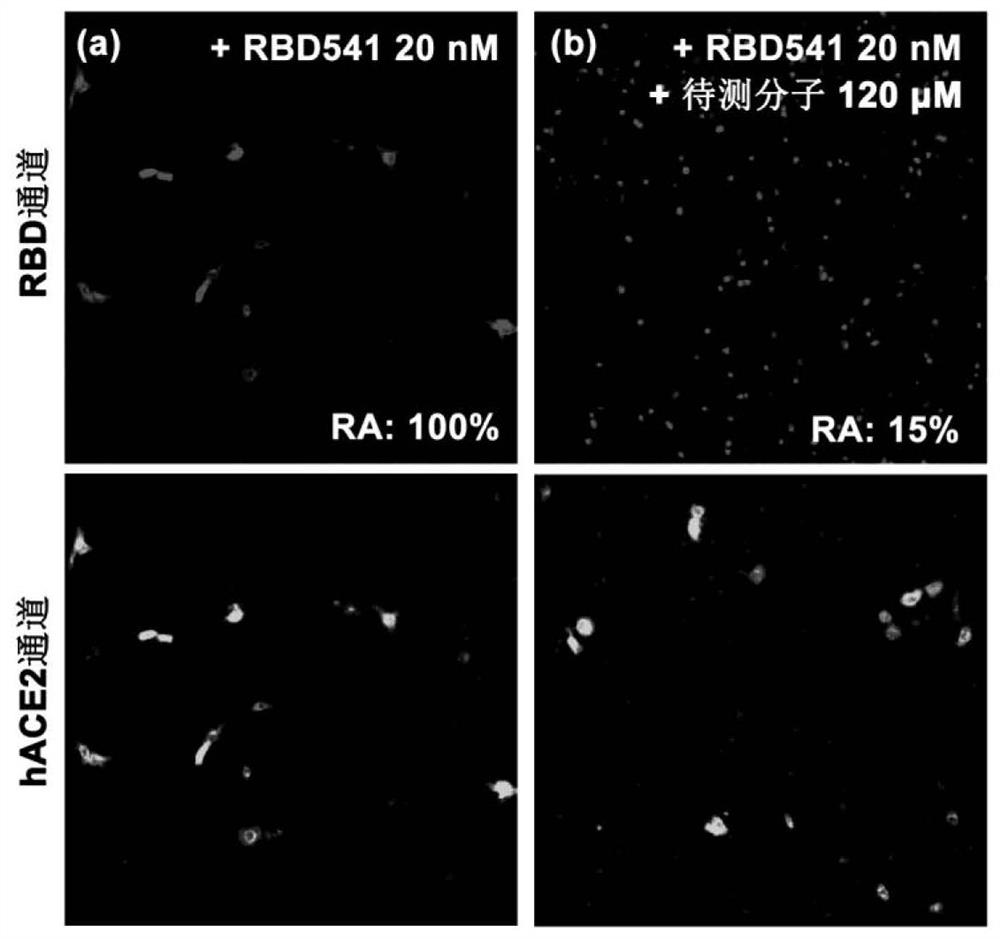
Screening method of small-molecule inhibitor of COVID-19 spinous process protein, active molecule screened by screening method and application of small-molecule inhibitor
PendingCN112750496AImprove screening efficiencyHigh enrichment rateMolecular designDrug referencesPharmacophoreChemical compound
The invention discloses a screening method of a COVID-19 spike protein inhibitor, an active molecule screened by the screening method and application of the inhibitor. The screening method comprises the following steps of: acquiring a three-dimensional structure of a cocrystal of COVID-19 spike protein and human angiotensin converting enzyme 2 from a database; acquiring active molecules and derivatives thereof of traditional Chinese medicinal materials from a traditional Chinese medicinal material active component database, and constructing a compound library of small molecules; and establishing a pharmacophore model, taking the pharmacophore model as a query formula, screening based on the matching degree value, and selecting a small molecule compound with the normalized matching value greater than 0.35. The effective S protein small-molecule inhibitor can be rapidly and effectively screened out according to the link that the novel coronavirus invades the S protein of the human cells to be combined with the human ACE2, and the screened-out inhibitor is definite in effect and has an application prospect in prevention or / and treatment of novel coronavirus pneumonia.
Owner:DALIAN UNIV OF TECH
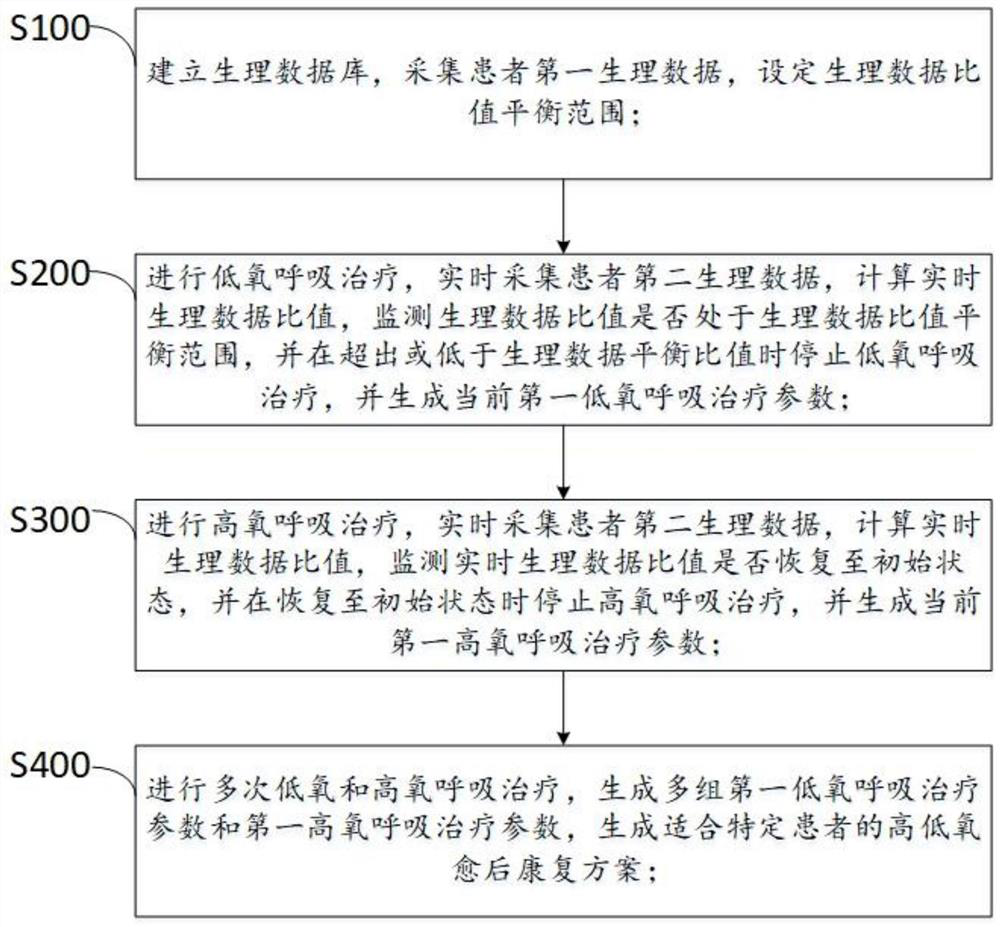
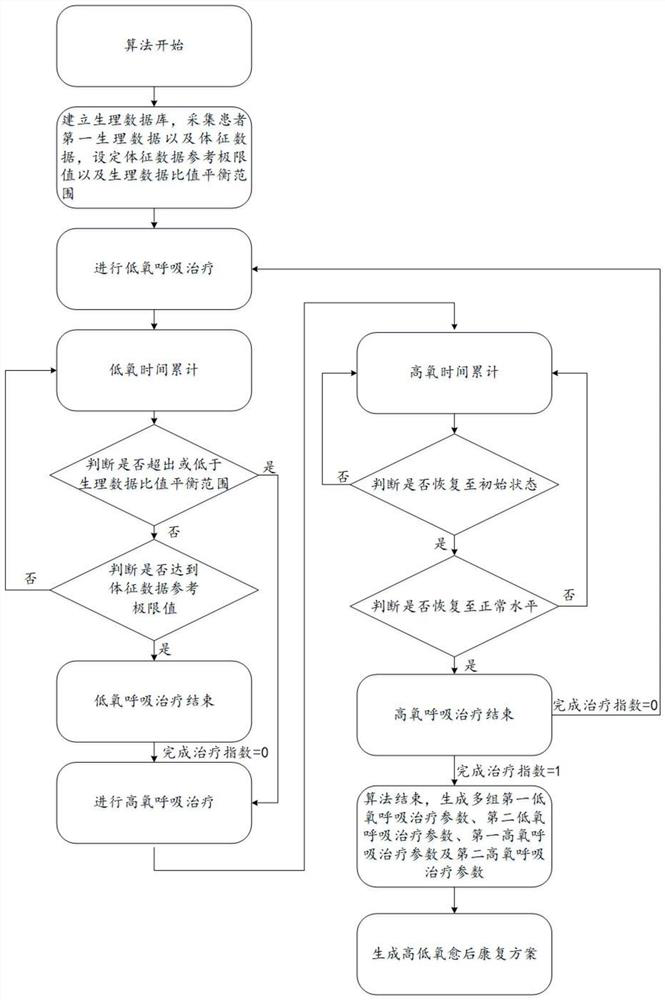
New coronal pneumonia rehabilitation method after cure based on intermittent high-low oxygen cardiopulmonary therapy system
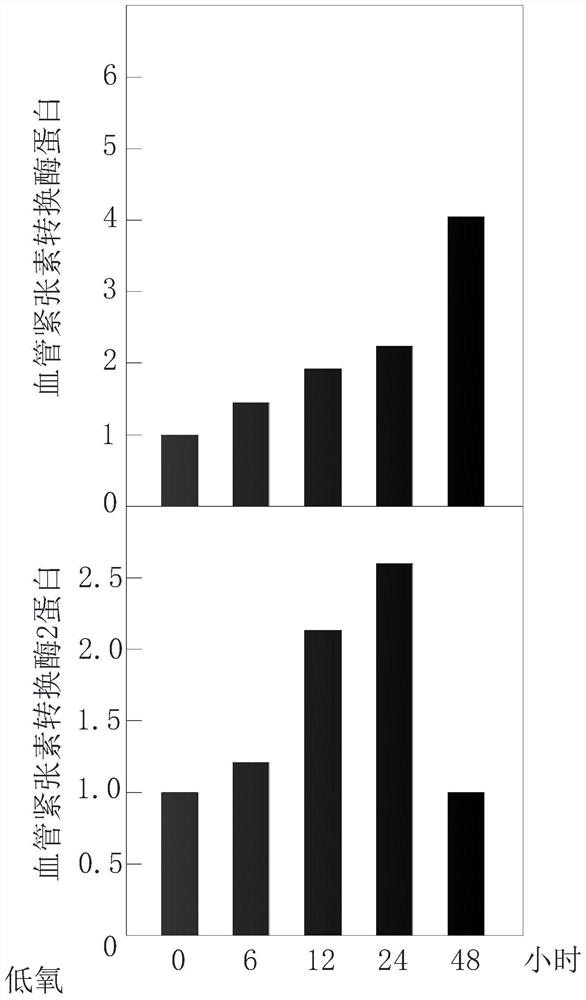
PendingCN112535787AImprove respiratory functionIncrease reservesRespiratorsElectrocardiographyAngiotensin-converting enzymePhysical medicine and rehabilitation
The invention relates to a new coronal pneumonia rehabilitation method after cure based on an intermittent high-low oxygen cardiopulmonary therapy system. The new coronal pneumonia rehabilitation method after cure comprises the following steps of establishing a physiological database, collecting first physiological data of a patient, and setting a physiological data ratio balance range; carrying out low-oxygen respiratory treatment, collecting second physiological data of the patient in real time, calculating a real-time physiological data ratio, stopping low-oxygen respiratory treatment and carrying out high-oxygen respiratory treatment if the real-time physiological data ratio exceeds or is lower than the physiological data balance ratio, and stopping high-oxygen respiratory treatment when the real-time physiological data ratio is recovered to an initial state; and performing high-low oxygen respiratory treatment for multiple times, generating multiple groups of first low-oxygen respiratory treatment parameters and first high-oxygen respiratory treatment parameters, and generating a high-low oxygen rehabilitation scheme after cure suitable for the patient, wherein the physiological data is the content of angiotensin converting enzyme and angiotensin converting enzyme 2 of the patient. By adopting the scheme, the patient can be subjected to rehabilitation treatment after new coronal pneumonia is cured, the lung function is improved, and the respiratory function is improved.
Owner:SHENZHEN SINO RUSSIA MEDICAL TECH CO LTD
Screening method and application of virus protein escape neutralizing antibody
PendingCN114736930AEfficient screeningSsRNA viruses positive-senseVirus peptidesSequence analysisSerine
The invention discloses a screening method and application of a virus protein escape neutralizing antibody, and the screening method comprises the following steps: constructing a random mutation library of virus protein, and introducing the random mutation library into a lentivirus plasmid vector to obtain a lentivirus plasmid library; the lentivirus plasmid library is used for packaging a pseudovirus, the pseudovirus is neutralized with a neutralizing antibody, cells expressing angiotensin converting enzyme 2 and cell serine protease are infected, cells expressing green fluorescence are screened out, DNA is extracted for sequence analysis, and a virus sequence capable of escaping the neutralizing antibody can be screened out. The screening system can be used for predicting the virus sequence of the escape neutralizing antibody, is simple, convenient and efficient, and provides a new platform for research of virus protein and hosts.
Owner:NANJING MEDICAL UNIV
Method for realizing fermentative expression of angiotensin converting enzyme 2 by using prokaryotic cell
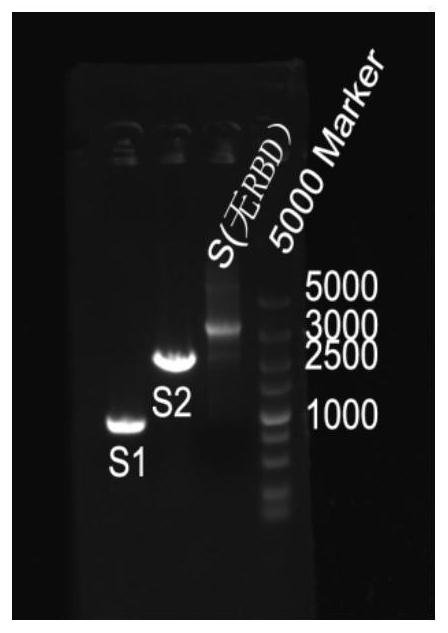
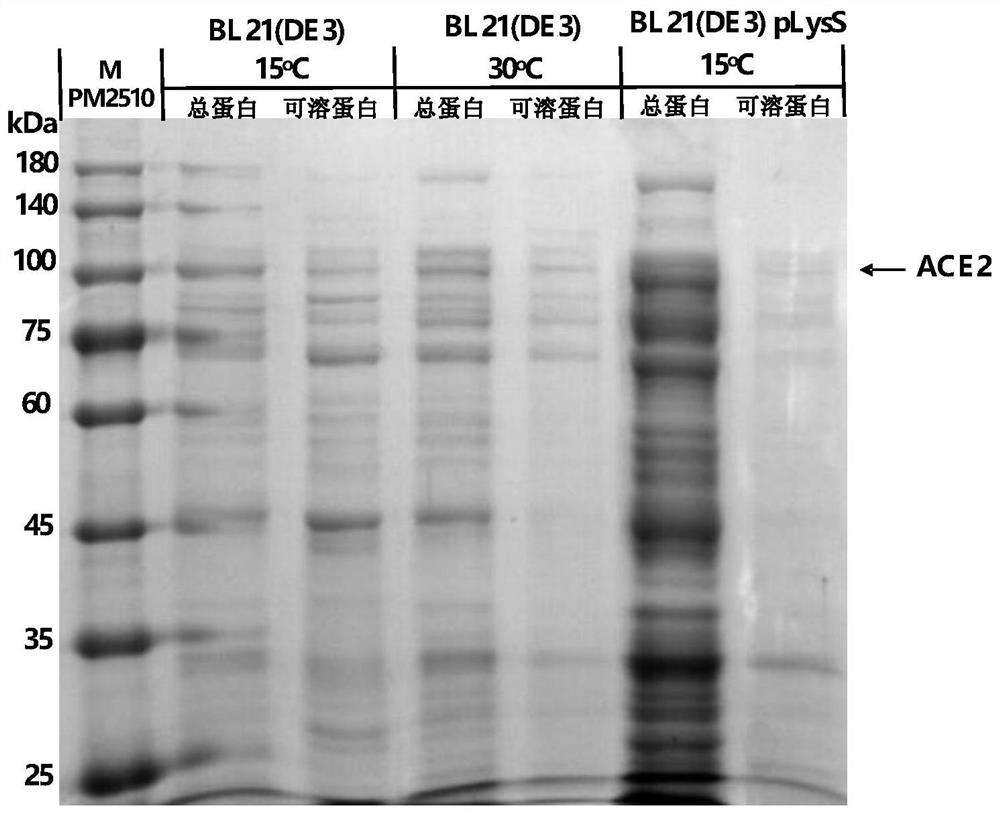
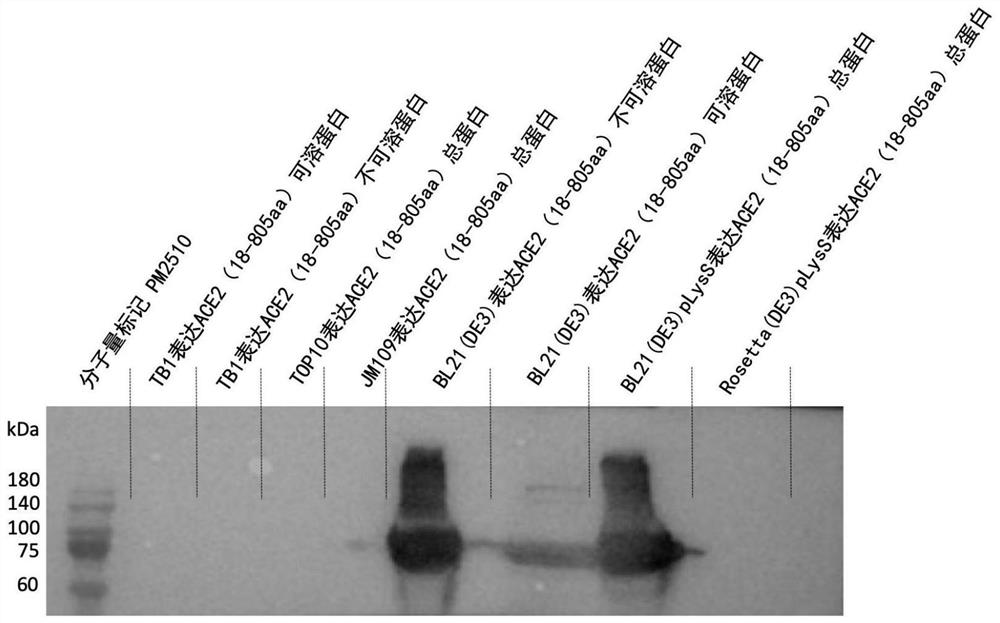
PendingCN112626099AGood water solubilityEasy extractionBacteriaMicroorganism based processesProkaryote organismsAngiotensin-converting enzyme 2
The invention relates to a plasmid containing angiotensin converting enzyme 2 (ACE2), a prokaryotic cell containing the plasmid and a method for realizing fermentative expression of ACE2 by using the prokaryotic cell as a host cell. The invention also relates to a method for producing ACE2 by using a prokaryote. The method comprises the following steps: constructing the plasmid containing a natural ACE2 sequence, knocking out the ACE2 sequence of a transmembrane region and an intracellular region, or further knocking out the ACE2 sequence of a part of extracellular region to serve as an exogenous gene; introducing the plasmid into a host prokaryote; culturing the host prokaryote, and expressing ACE2 in a culture; and extracting and purifying ACE2 from the culture.
Owner:TSINGHUA UNIV +1
Application of small-molecule inhibitor in prevention and treatment of respiratory viral pneumonia
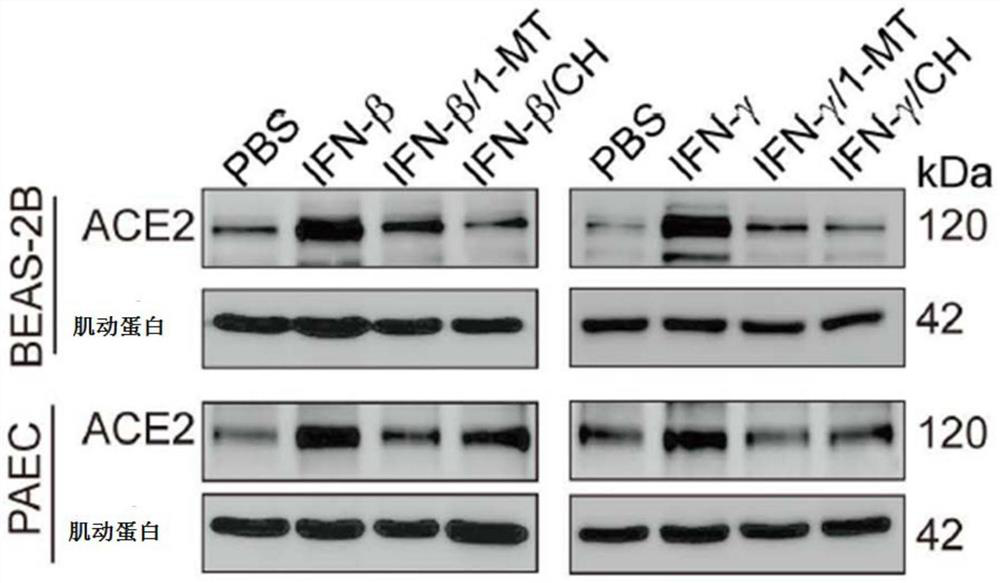
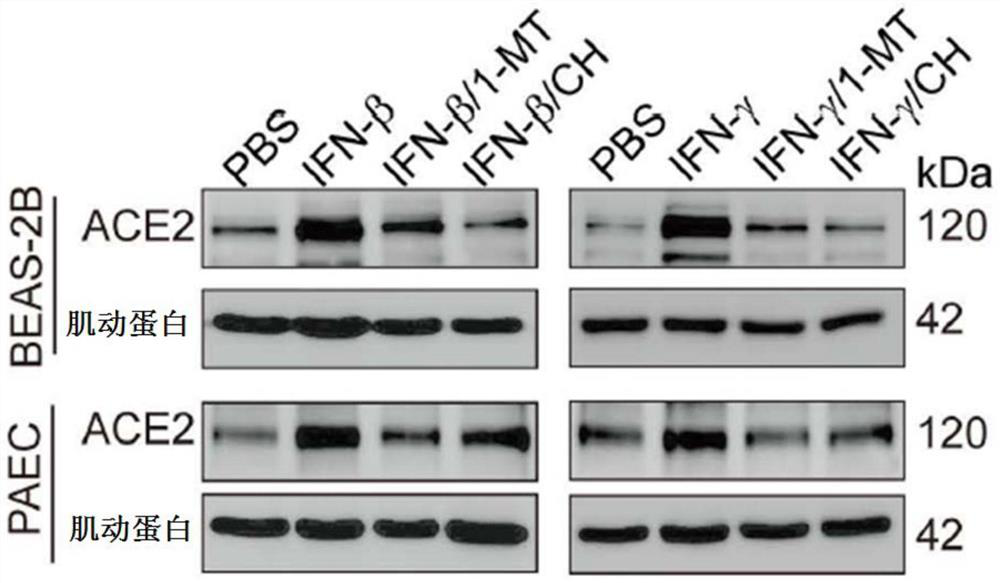
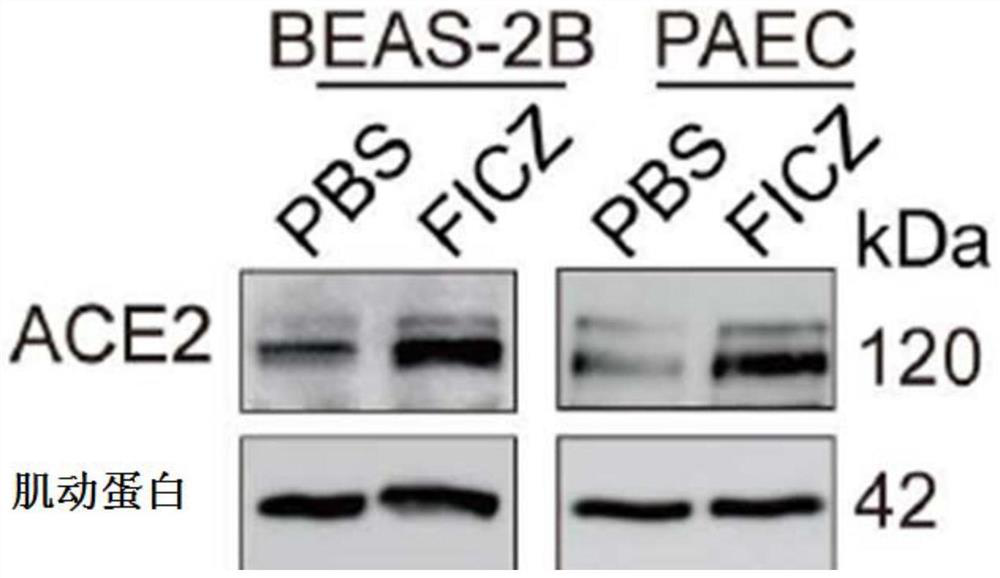
The invention provides application of a small-molecule inhibitor in prevention and treatment of respiratory viral pneumonia. Specifically, the invention relates to a novel application of an AhR inhibitor in treating or improving virus infection. The inhibitor for treating virus infection provided by the invention can effectively inhibit expression of angiotensin converting enzyme 2 so as to prevent virus from infecting lung tissues, and the AhR inhibitor is expected to become a potential medicine for preventing and treating lung diseases caused by virus infection.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
ACE2 mutant as well as mutation method and application thereof
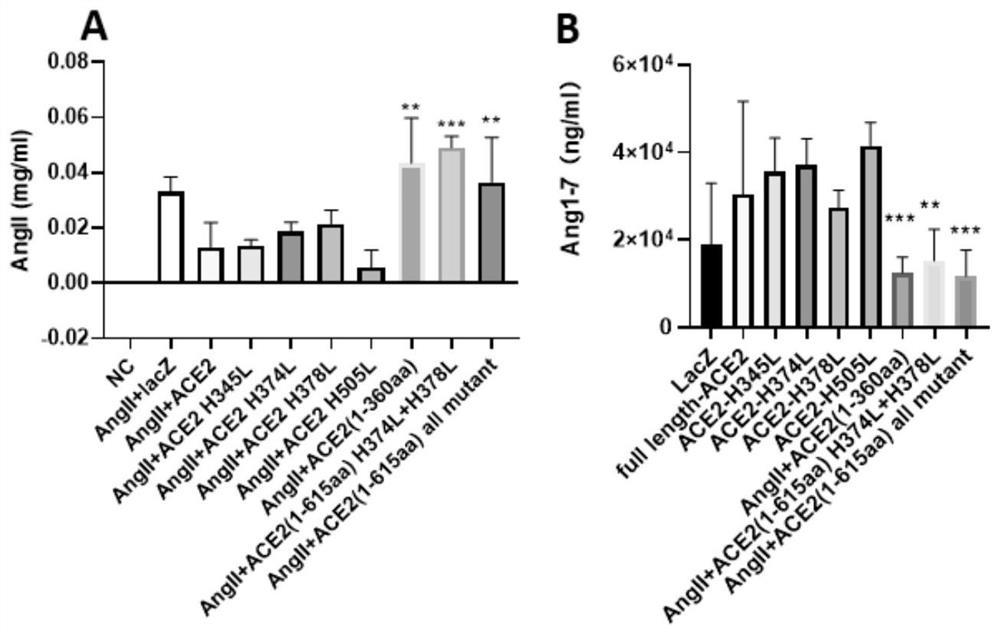
The invention relates to a mutant of an angiotensin converting enzyme 2 (ACE2) protein or an extracellular domain thereof, which is a mutant obtained by mutating one or more histidines (namely histidines at any one or more positions of the 345th site, the 374th site, the 378th site and the 505th site of the N end) in zinc ion dependent active sites of the N end of the ACE2 extracellular domain into non-polar amino acids. As bait protein of the coronavirus, the mutant can play a role in trapping the coronavirus outside cells and prevent the virus from entering the cells; and meanwhile, the level of angiotensin II cannot be degraded due to mutation of a specific site, so that the simultaneous accompanying side effects such as RAAS disorder and myocardial fibrosis caused by rapid increase of a large amount of ACE2 or extracellular fragments thereof are avoided.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com