High-affinity human angiotensin converting enzyme 2 (ACE2) mutant and application thereof
A mutant, T27W technology, applied in the field of bioengineering, can solve the problems that affect the therapeutic effect, cannot effectively block the combination of the virus and the airway epithelial cell membrane receptor ACE2, and low affinity, and achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
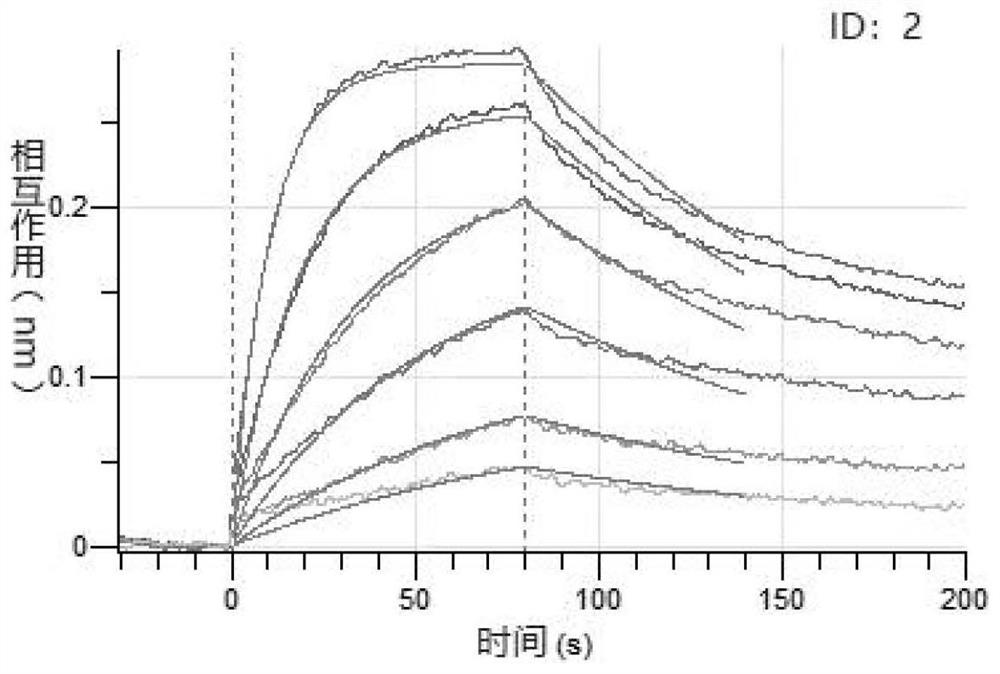
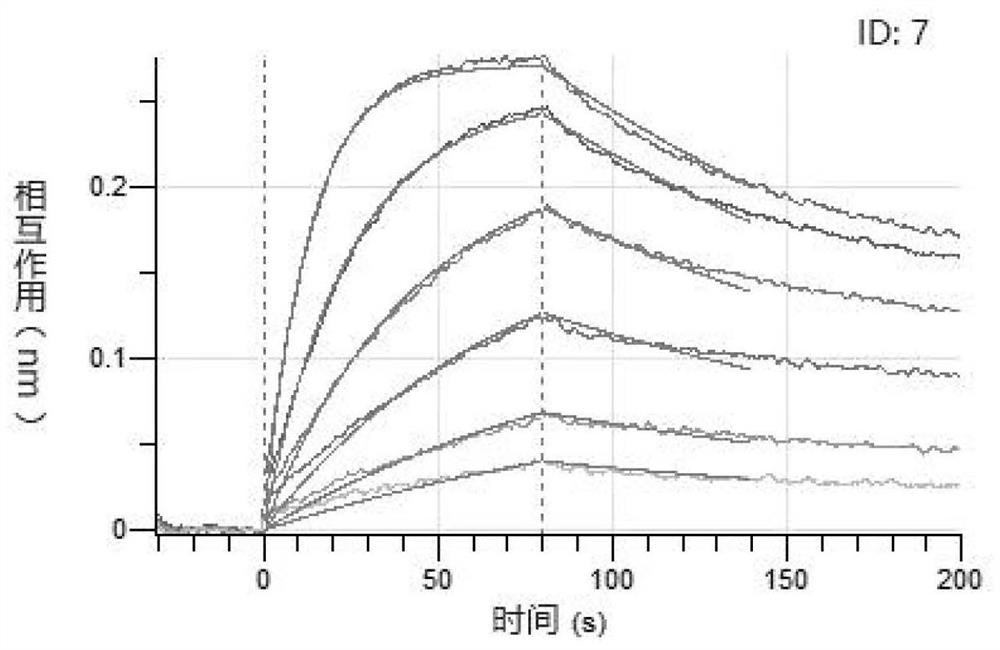
Image
Examples
Embodiment 1
[0064] Example 1 Angiotensin-converting enzyme 2 (ACE2) mutant design
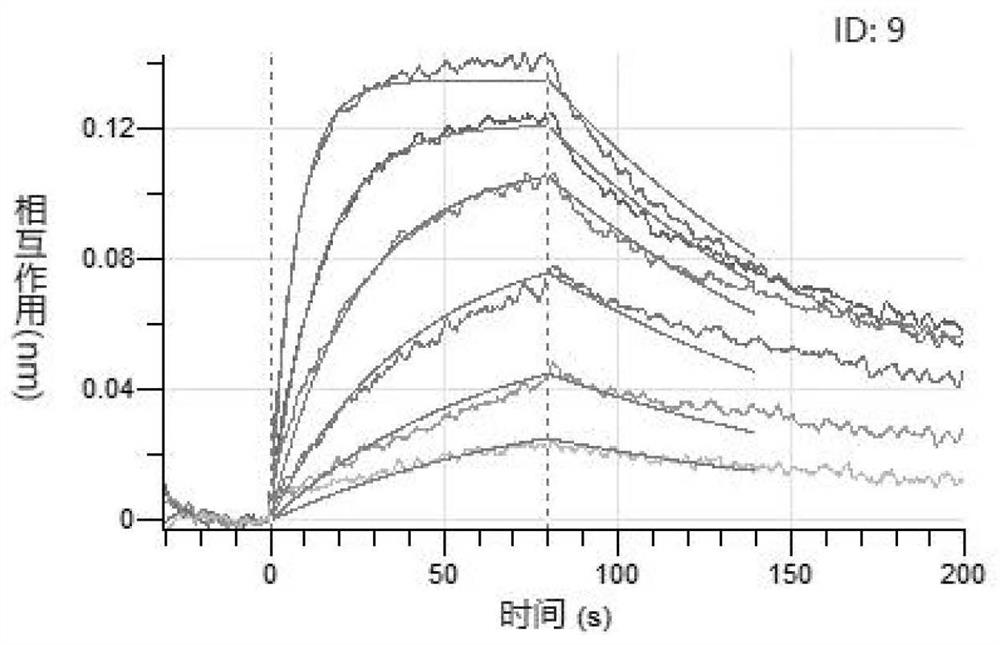
[0065] Two interacting proteins are bound by hydrogen bonds, ionic bonds, van der Waals forces and hydrophobic interactions between their amino acid side chains. By observing and analyzing the structures of ACE2 and SARS-CoV-2 (PDB ID: 6M18), the present invention has designed a series of ACE2 mutants (the amino acid sequences thereof are respectively as SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO : 3, SEQ ID NO: 4, SEQ ID NO: 12, SEQ ID NO: 20, SEQ ID NO: 22, SEQ ID NO: 24, SEQ ID NO: 26, SEQ ID NO: 28, SEQ ID NO: 30, SEQ shown in ID NO:32 and SEQ ID NO:34). The mutated ACE2 variant is expressed as a dimer (i.e. ACE2-Fc Protein, its sequence is as SEQ ID NO:5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:8, SEQ ID NO:13, SEQ ID NO:21, SEQ ID NO:23, SEQ ID NO :25, SEQ ID NO:27, SEQ ID NO:29, SEQ ID NO:31, shown in SEQ ID NO:33 and SEQ ID NO:35).
[0066] Table 1 ACE2 mutants and ACE2-Fc protein
[0067]
Embodiment 2
[0068] Example 2 The plasmid construction of the S protein of the new coronavirus SARS-CoV-2
[0069] The plasmid p3XFLAG-CMV14-2019nCoV-S expressing the S protein of the new coronavirus SARS-CoV-2:
[0070] The DNA sequence (SEQ ID NO: 11) of the S protein ORF of SARS-CoV-2 synthesized by genes was digested with restriction DNA endonucleases HindIII and XbaI, and the plasmid vector p3XFLAG was digested with the same restriction enzymes at the same time -5CMV14 (Sigma, Cat. No. E4901), the S protein ORF with cohesive ends obtained after enzyme digestion and the plasmid vector fragment were ligated with T4 ligase, and transformed into E. coli competent cells to obtain the plasmid p3XFLAG-CMV14-2019nCoV-S (DOI : https: / / doi.org / 10.1371 / journal.pone.0076469).
Embodiment 3
[0071] Example 3 Production and purification of wild-type and mutant ACE2-Fc dimers
[0072] 3.1 Cell culture and transient transfection
[0073] CHO-3E7 cells were grown in serum-free FreeStyle™ CHO expression medium (Life Technologies, Carlsbad, California, USA). Cells were incubated on an orbital shaker (VWR Scientific, Chester, PA) at 37°C, 5% CO 2held in an Erlenmeyer flask (Corning Inc., Acton, MA). On the day of transfection, DNAs encoding ACE2-Fc proteins of SEQ ID NO: 5-8, SEQ ID NO: 13, 21, 23, 25, 27, 29, 31, 33 and 35 (sequences such as SEQ ID NO: 16- 19) and PEI (Polysciences, Eppelheim, Germany) were mixed in a certain ratio, and then added to the flask together with the cells to be transfected. On day 5 of transfection, about 1 ml of supernatant was collected for detection of expression level. The supernatant collected on day 6 was used for further purification.
[0074] 3.2 Purification and analysis
[0075] Centrifuge the cell culture fluid, then filter....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com