Novel coronavirus neutralizing antibody detection test paper
A coronavirus and antibody detection technology, applied in the field of biomedical detection, can solve problems such as time-consuming, low detection specificity, and complicated detection operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Novel coronavirus neutralizing antibody detection test paper
[0058] This embodiment provides a novel coronavirus neutralizing antibody test strip, including:
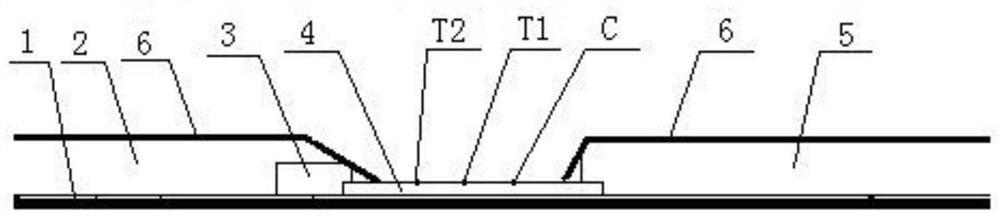
[0059] Such as figure 1 As shown, it includes a substrate 1 and a sample pad 2, a colloidal gold adsorption pad 3, an antibody carrying film 4 and a water absorbing pad 5 are overlapped and overlapped on the substrate 1 in sequence; the antibody carrying film 4 is provided with a corresponding The detection line T1 , the detection line T2 and the quality control line C are spaced apart, the detection line T2 is close to the colloidal gold adsorption pad 3 , and the quality control line C is close to the water absorption pad 5 . The colloidal gold adsorption 3 is coated with the receptor binding domain RBD antigen of the new coronavirus S protein labeled with colloidal gold and the antibody unrelated to the new coronavirus labeled with colloidal gold. In this embodiment, the antibody unrelated to the ...
Embodiment 2
[0062] This embodiment provides a method for preparing the novel coronavirus neutralizing antibody detection test paper in Example 1, and the preparation steps of the test paper are as follows:
[0063] 1. Preparation of antibody-carrying membrane:
[0064] (1) Select a nitrocellulose membrane with a pore size of 3 μm to 10 μm, and cut the membrane into specifications with a width of 2.0 cm and a length of 30.5 cm as required, for later use.
[0065] (2) Use 0.1M, pH8.0 Tris-HCL buffer solution to prepare the mouse anti-human IgG monoclonal antibody used for the detection line coating. ml, the concentration of ACE-2 is 1.5 to 2.0 mg / ml, in this example it is 1.8 mg / ml, and the goat anti-rabbit IgG used for quality control line coating is prepared with 0.85% by mass sodium chloride buffer For polyclonal antibodies, the antibody concentration is 2.0-2.5 mg / ml, which is 2.2 mg / ml in this embodiment.
[0066] (3) Select the antibody-coated surface of the nitrocellulose membrane ...
Embodiment 3
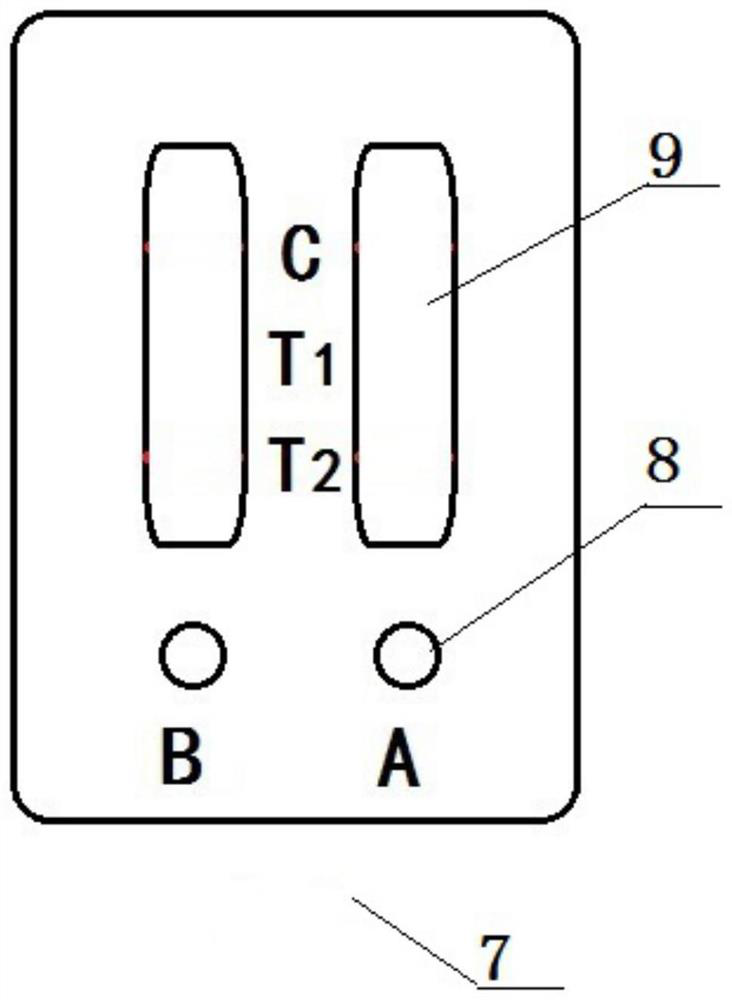
[0080] This embodiment provides a novel coronavirus neutralizing antibody detection device, such as figure 2 As shown, a housing 7 is included, and two novel coronavirus neutralizing antibody detection test papers of embodiment 1 are arranged side by side inside the housing 7 . Along the chromatographic direction of the novel coronavirus neutralizing antibody detection test paper on the housing 7, a sample hole 8 is set at the corresponding sample pad, and an observation port 9 is set at the detection line T2, the detection line T1 and the quality control line C .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com