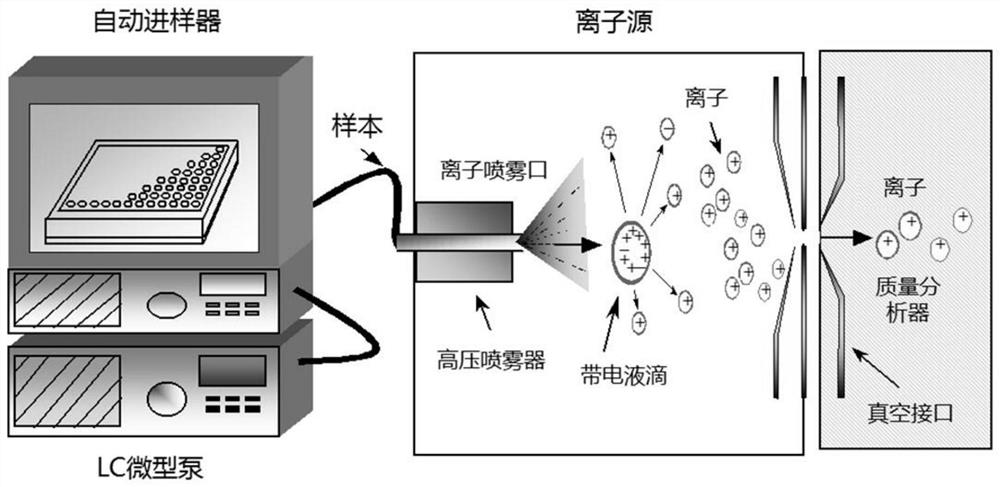
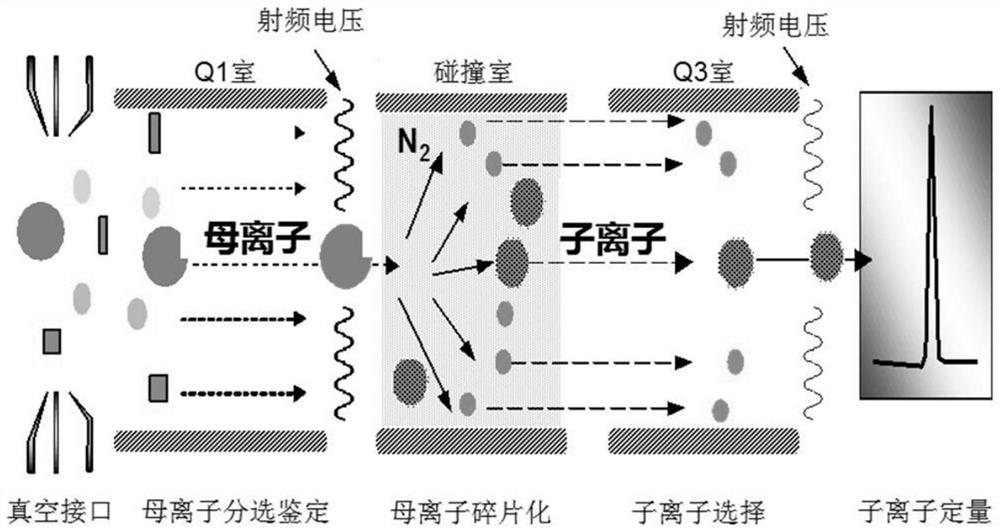
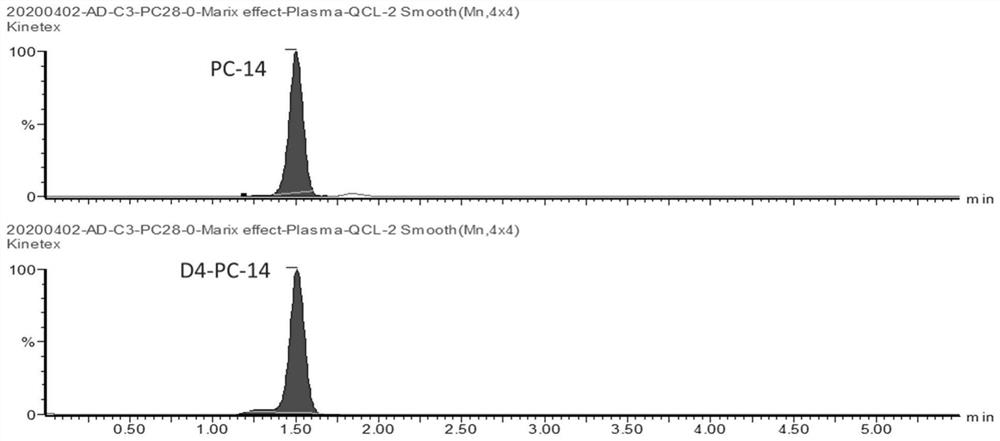
Biomarker combination for evaluating Alzheimer's disease and application and kit thereof
A technology of biomarkers and kits, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of no blood markers, disappointing results, no detection kits for detecting AD with blood markers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] S2. Preparation steps of quality control working solution and internal standard working solution:
[0071] The above-mentioned high-concentration quality control products and low-concentration quality control products can be directly used as quality control product working solutions; the mixture of the above-mentioned internal standard products can be directly used as internal standard working solutions;
[0072] S3. Sample pretreatment:
[0073] Precisely transfer 135 μL of sample extract to each well of a 96-well plate, and add 20 μL of the plasma sample to be tested, blank control, negative control, positive control, calibrator working solution, and quality control working solution to different wells , and then add 45 μL of internal standard working solution (three mixed internal standards: d4-PC-14, 2 h 3 -C-3, DHBA, that is, the above-mentioned internal standard mixed solution), sealed, mixed for 2 minutes, left at room temperature for 5 minutes, centrifuged at 4...
Embodiment 1
[0119] This embodiment is the composition and detection method of the kit of the present invention.
[0120] One, the test kit of the present embodiment comprises:
[0121] Internal standard mixed solution: d4-PC-14, the concentration is 600ng / ml (solvent: methanol / acetonitrile 9:1); 2 h 3 -C-3, the concentration is 20ng / ml (solvent: methanol / acetonitrile 9:1); DHBA, the concentration is 50μg / mL (solvent: 80% methanol water);
[0122] High-concentration calibrator mixed solution: PC-14 (DMPC), with a concentration of 30 μg / ml (solvent: methanol / acetonitrile 9:1); C-3, with a concentration of 8 μg / ml (solvent: methanol / acetonitrile 9 : 1); Ach and GABA, use concentration is 2.5 μ g / ml (solvent is: 80% methanol water); 5-HIAA and Glu, use concentration is 12.5 μ g / ml (solvent is: 80% methanol water);
[0123] Multi-concentration quality control product mixed solution: (1) low quality control product mixed solution: PC-14 (DMPC), the use concentration is 1.8μg / ml (solvent: met...
Embodiment 2
[0212] This embodiment is the composition and detection method of the kit of the present invention.
[0213] One, the test kit of the present embodiment comprises:
[0214] Internal standard: d4-PC-14, the concentration is 600ng / ml, 2 h 3 -C-3, the concentration is 20ng / ml; DHBA, the concentration is 50μg / mL;
[0215] High-concentration calibrator mixed solution: PC-14 (DMPC), used at a concentration of 30 μg / ml; C-3, used at a concentration of 8 μg / ml; Ach and GABA, used at a concentration of 2.5 μg / ml; 5-HIAA and Glu, The concentration used is 12.5μg / ml;
[0216] Multi-concentration quality control mixed solution: (1) Low quality control mixed solution: PC-14 (DMPC), the use concentration is 1.8 μg / ml; C-3, the use concentration is 0.48 μg / ml; Ach and GABA, The concentration used is 0.03 μg / ml; 5-HIAA and Glu, the concentration used is 1.8 μg / ml; (2) High-quality control product mixed solution: PC-14 (DMPC), the concentration used is 22.5 μg / ml; C-3 , the concentration ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com