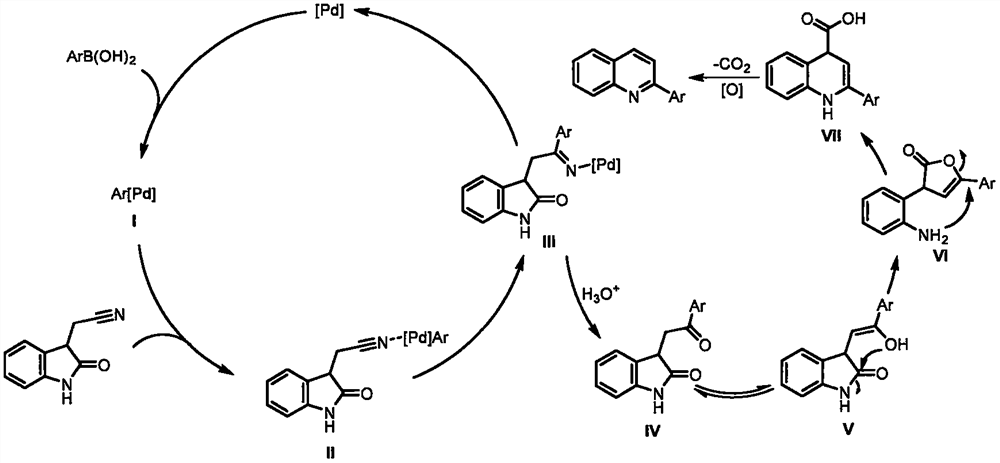
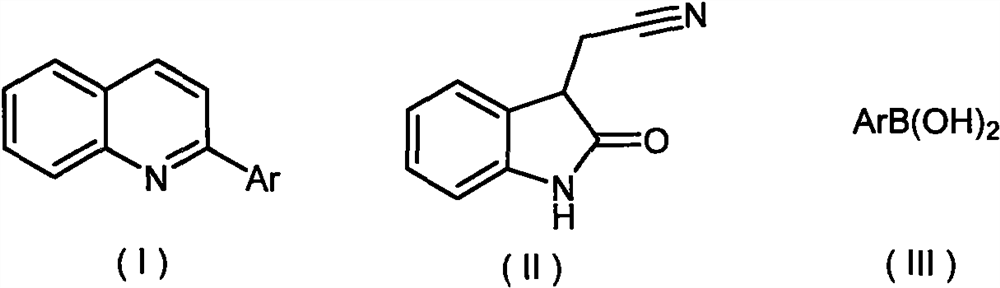
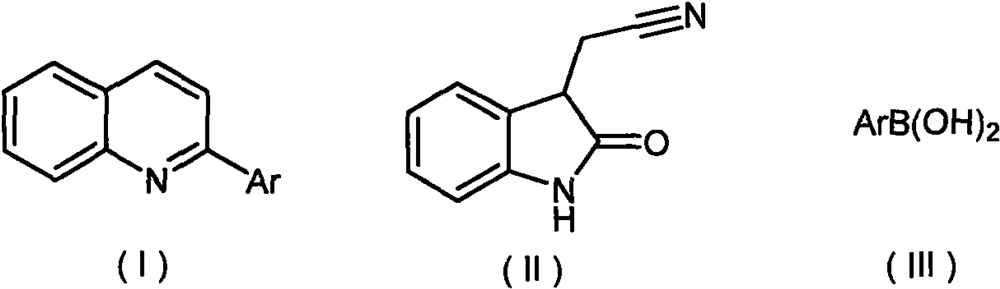
Synthesis method of 2-aryl quinoline compound
A synthesis method and arylquinoline technology, applied in the direction of organic chemistry, etc., can solve the problems of low yield, poor tolerance of substrate functional groups, and complicated operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the synthesis of 2-phenylquinoline
[0030]
[0031] In a dry and clean reactor, add 15mL solvent water and 5mL solvent tetrahydrofuran, then add the above formula (II) compound, formula (III) compound, palladium acetate, bipyridine, p-toluenesulfonic acid successively, so that the molar ratio is 1 : 2: 0.05: 0.1: 8, wherein the compound of formula (II) is 3 mmol. The reaction system was stirred and reacted at 80° C. for 24 hours. After the reaction, the solvent in the reactor was removed by a rotary evaporator, and the residue was purified by column chromatography with 200-300 mesh silica gel to obtain the target product as a yellow oil with a yield of 86%.
[0032] NMR data: 1 H NMR (500MHz, CDCl 3 )δ8.22-8.18(m, 4H), 7.87(d, J=8.5Hz, 1H), 7.83(d, J=8.0Hz, 1H), 7.76-7.73(m, 1H), 7.56-7.52(m , 3H), 7.50-7.47(m, 1H); 13 C NMR (125MHz, CDCl 3)δ157.3, 148.2, 139.6, 136.7, 129.7, 129.6, 129.3, 128.8, 127.5, 127.4, 127.1, 126.2, 118.9.
Embodiment 2
[0033] Embodiment 2: the synthesis of 2-(o-tolyl) quinoline
[0034]
[0035] In a dry and clean reactor, add 15mL solvent water and 5mL solvent tetrahydrofuran, then add the above formula (II) compound, formula (III) compound, palladium acetate, bipyridine, p-toluenesulfonic acid successively, so that the molar ratio is 1 : 2: 0.06: 0.12: 8, wherein the compound of formula (II) is 3 mmol. The reaction system was stirred and reacted at 90° C. for 24 hours. After the reaction, the solvent in the reactor was removed by a rotary evaporator, and the residue was purified by column chromatography with 200-300 mesh silica gel to obtain the target product as a yellow oil with a yield of 75%.
[0036] NMR data: 1 H NMR (500MHz, CDCl 3 )δ8.23-8.18 (m, 2H), 7.87 (d, J=8.5Hz, 1H), 7.77-7.74 (m, 1H), 7.59-7.54 (m, 2H), 7.53-7.51 (m, 1H) , 2.43(s, 3H); 13 C NMR (125MHz, CDCl 3 )δ160.2, 147.8, 140.6, 136.1, 136.0, 130.8, 129.7, 129.6, 129.5, 128.5, 127.5, 126.7, 126.4, 126.0, 122.3,...
Embodiment 3
[0037] Embodiment 3: the synthesis of 2-(p-tolyl) quinoline
[0038]
[0039] In a dry and clean reactor, add 15mL solvent water and 5mL solvent tetrahydrofuran, then add the above formula (II) compound, formula (III) compound, palladium acetate, bipyridine, p-toluenesulfonic acid successively, so that the molar ratio is 1 : 2: 0.05: 0.1: 10, wherein the compound of formula (II) is 3 mmol. The reaction system was stirred and reacted at 90° C. for 24 hours. After the reaction, the solvent in the reactor was removed by a rotary evaporator, and the residue was purified by column chromatography with 200-300 mesh silica gel to obtain the target product as a yellow oil with a yield of 88%.
[0040] NMR data: 1 H NMR (400MHz, CDCl 3 )δ8.19(d, J=8.4Hz, 2H), 8.08(d, J=8.4Hz, 2H), 7.86(d, J=8.4Hz, 1H), 7.83-7.80(m, 1H), 7.75- 7.71(m, 1H), 7.54-7.50(m, 1H), 7.35(d, J=8.0Hz, 2H), 2.45(s, 3H); 13 C NMR (125MHz, CDCl 3 )δ157.3, 148.2, 139.4, 136.7, 136.7, 129.6, 129.6, 129.5, 127.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com