Functional single-chain cyclic poly (beta-amino ester) as well as preparation method and gene delivery drug application thereof
A functional, aminoester technology, applied in the field of functional single-chain cyclic polymer and its preparation, gene delivery drug application field, can solve the problems of no way, limited clinical approval and large-scale application, achieve narrow PDI, charge The effect of easy adjustment of density and composition, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
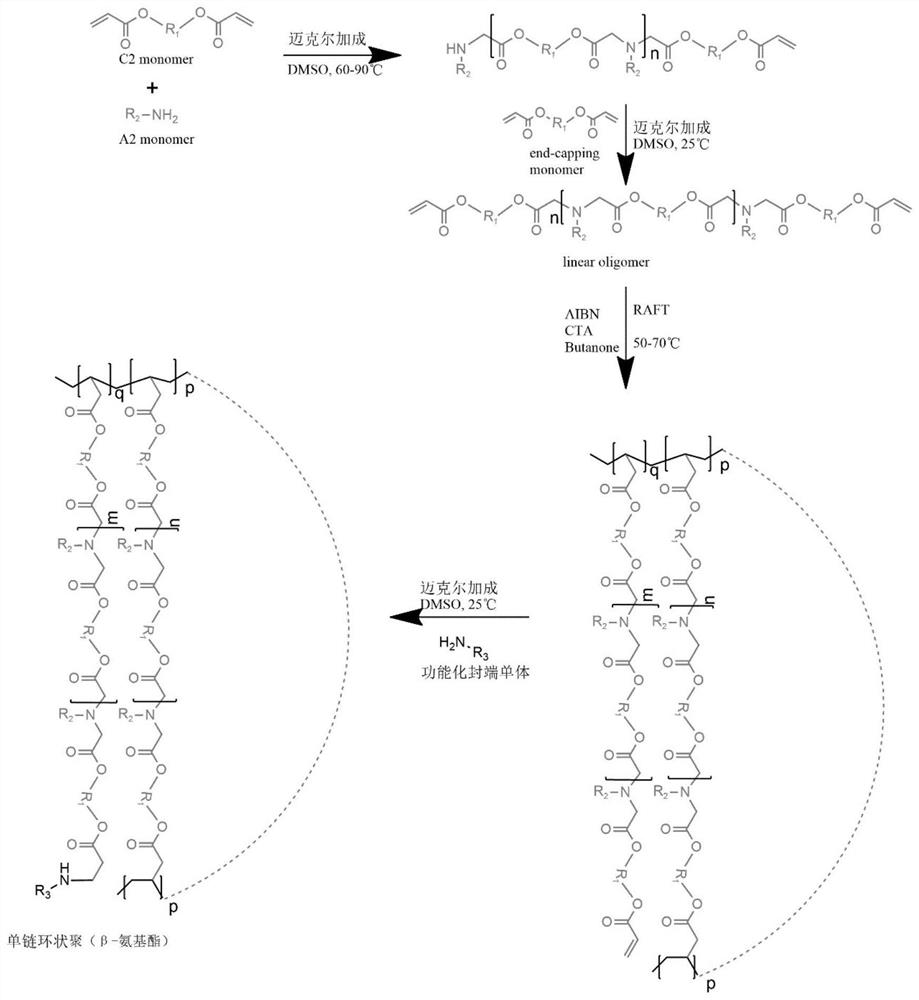
[0038] A synthetic method of a class of functional single-chain cyclic poly(β-amino ester) disclosed by the present invention, the synthetic route can be found in figure 1 , including the following steps:
[0039]1) A linear poly(β-amino ester) with a double bond is obtained through a Michael addition reaction between a diacrylate monomer and a small molecule organic amine;
[0040] 2) The linear poly(β-amino ester) with double bond prepared in step 1) is subjected to reversible addition-fragmentation chain transfer polymerization to obtain a single-chain polycyclic poly(β-amino ester) with controllable structure and composition ;
[0041] 3) Adding a functional end-capping agent to the single-chain polycyclic poly(β-amino ester) prepared in step 2) for functional end-capping treatment to prepare a functional single-chain cyclic poly(β-amino ester).
[0042] Specifically, the following steps are included:
[0043] 1) Add a certain amount of diacrylate monomer and small mole...
Embodiment 1
[0064] The reaction ratio of 2,2-dithiodiethanol diacrylate and dodecylamine is 2:1, react at 60°C for 5h; add 0.5 equivalent of 2,2-dithiodiethanol diacrylate, After 48 hours of reaction, the obtained low molecular weight linear poly(β-amino ester) had a molecular weight of 3000 Da.
[0065] Low molecular weight linear poly(β-amino ester), chain transfer agent and AIBN reaction feed ratio is 300:1:1, react at 50°C for 50h, then add 4 equivalents of N-(2-aminoethyl)morpholine, After reacting at 25°C for 48 hours, the obtained single-chain polycyclic poly(β-amino ester) had a molecular weight of 30,000 Da.
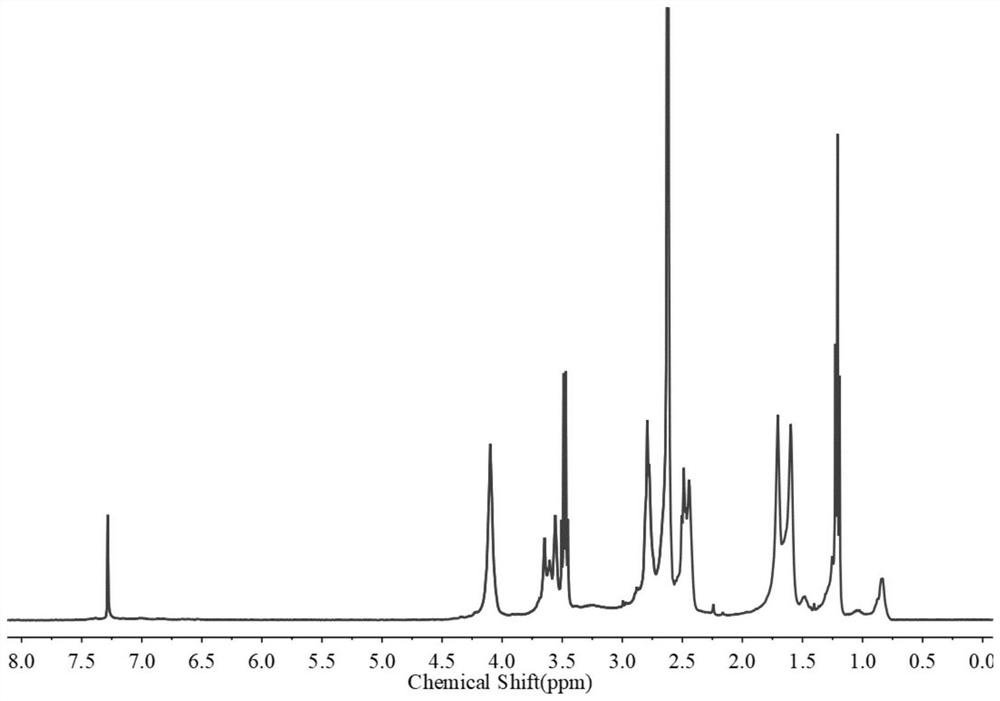
[0066] figure 2 It is a solution state in which the single-chain polycyclic poly(β-amino ester) with a molecular weight of 30,000 Da prepared in this example is dissolved in dimethyl sulfoxide. image 3 The H NMR spectrum of the single-chain polycyclic poly(β-amino ester) with a molecular weight of 30,000 Da prepared for this example.
[0067] see Figure 4 , Figure ...
Embodiment 2
[0069] The reaction ratio of bisphenol A polyoxyethylene ether diacrylate and dodecylamine is 0.5:1, react at 60°C for 2h; add 1 equivalent of bisphenol A polyoxyethylene ether diacrylate, react at 25°C for 48h , to obtain a linear poly(β-amino ester) with a molecular weight of 2800 Da.
[0070] Low molecular weight linear poly(β-amino ester), chain transfer agent and AIBN reaction ratio is 200:5:1, react at 50℃ for 96h, then add 5 equivalents of 1,11-diamino-3,6,9 - Trioxaundecane, reacted at 25° C. for 48 hours, and the molecular weight of the obtained single-chain polycyclic poly(β-amino ester) was 40000 Da.
[0071] see Figure 4 , Figure 4 (b) is the purified gel permeation chromatography (GPC) curve of the single-chain polycyclic poly(β-amino ester) with a molecular weight of about 40,000 Da prepared in this example.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com