Application of polycyclic aryl compound in preparation of antifungal drugs
An antifungal drug and polycyclic aromatic group technology, which is applied in antifungal agents, drug delivery, and resistance to vector-borne diseases, etc., can solve the problems of antibacterial drug resistance, limited drug selection, and low bioavailability, and achieve The effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
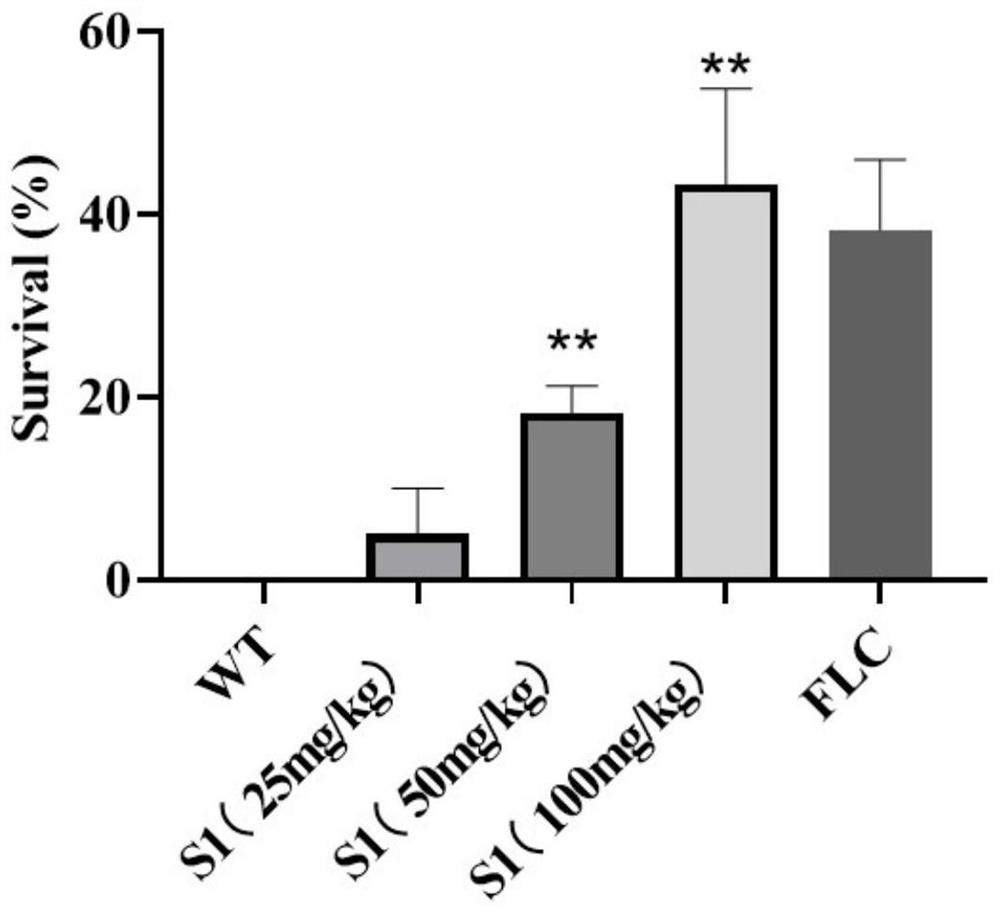
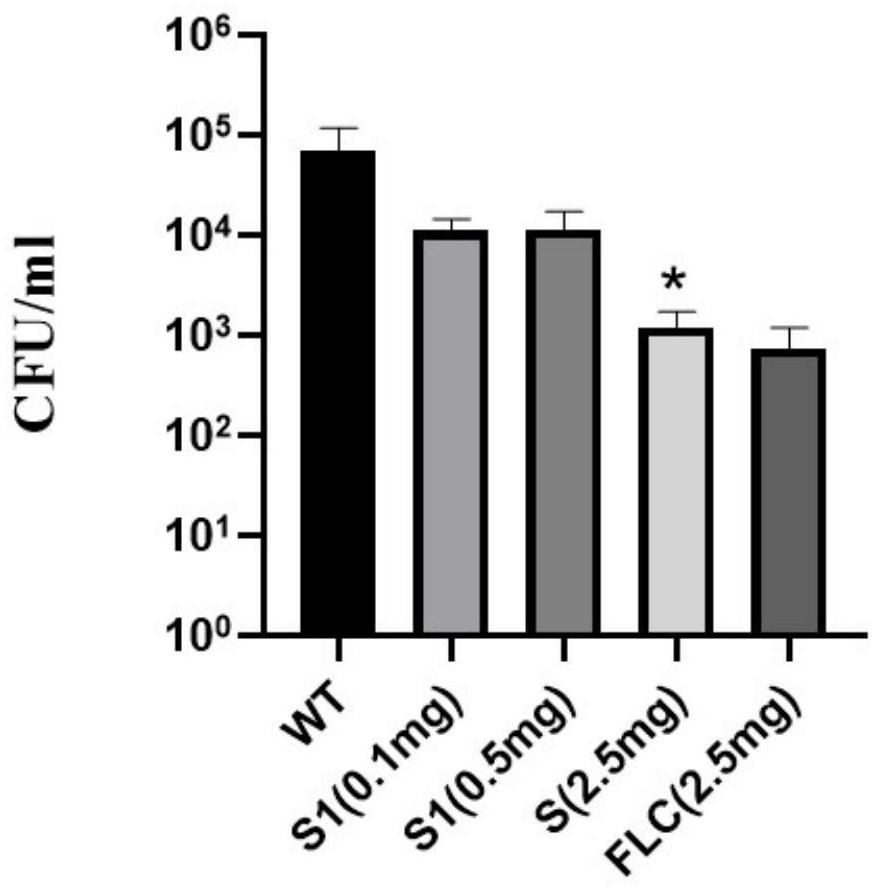
Examples
Embodiment 1
[0033] The synthesis of embodiment 1 compound 1-3
[0034]
[0035] Compound 1-1 (5.00g, 21.8mmol, 1.00eq) was dissolved in dichloromethane (25mL) at room temperature, and then compound 1-2 (3.54g, 26.2mmol, 3.13mL, 1.20eq) was added. After stirring and reacting for 12 hours, the reaction solution was filtered and concentrated to obtain a crude product. The obtained crude product was purified by silica gel column chromatography (DCM:MeOH=8:1) to obtain white solid compound 1-3.
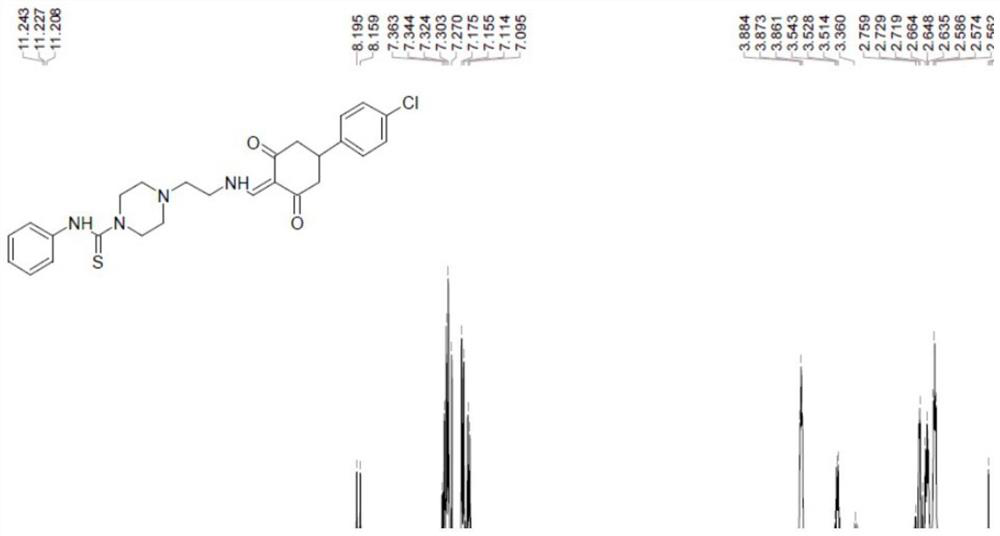
[0036] Compounds 1-3: 1 H-NMR (400MHz, CDCl 3 ), δ7.42(s,1H),7.34-7.30(t,J=7.8Hz,2H),7.16(s,1H),7.14-7.10(t,J=7.6Hz,2H),4.91(s, 1H) 3.83-3.80(t, J=4.8Hz, 4H), 3.22-3.21(d, J=5.2Hz, 2H), 2.50-2.46(m, 6H), 1.45(s, 9H); LC-MS m / z.
Embodiment 2
[0037] The synthesis of embodiment 2 compound 1-4
[0038]
[0039] Compound 1-3 (12.0g, 32.9mmol, 1.00eq) was dissolved in methanol (60.0mL), and hydrochloric acid methanol solution (32.9mmol, 48.0mL, 1.00eq) was added at zero temperature. Subsequently, the reaction was raised to normal temperature for reaction. After reacting for 16 hours, the reaction solution was concentrated under reduced pressure to obtain crude hydrochloride compound 1-4 (13.1 g) as a white solid.
[0040] Compound 1-4: LC-MS m / z 265.2[M+H] + .
Embodiment 3
[0041] The synthesis of embodiment 3 compound 1-6
[0042]
[0043] At room temperature, compound 1-5 (8.00g, 35.9mmol, 1.00eq) was dissolved in N,N-dimethylformamide (100mL), and then N,N-dimethylformamide dimethyl acetal was added (35.9 g, 301 mmol, 40.0 mL, 8.38 eq). After stirring and reacting for 2 hours, the reaction solution was filtered to obtain a filtrate. The filtrate was spin-dried and concentrated under reduced pressure to obtain light yellow solid compound 1-6 (8.50 g, 30.6 mmol, yield 85.2%).
[0044] Compounds 1-6: 1 H NMR (400MHz, CDCl 3 ),δ8.08(s,1H),7.32-7.30(m,2H),7.19-7.17(d,J=8.4Hz,2H),3.43(s,3H),3.37-3.32(m,1H), 3.22(s,3H),2.77-2.62(m,4H); LC-MS m / z 278.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com