Methods for production of ergothioneine
A technology of ergothioneine and hydroxysulfanyl, applied in the direction of microorganism-based methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve problems such as cost-effectiveness, environmental impact, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0609] Example 1 - Materials and methods
[0610] Strains, chemicals, synthetic genes, services
[0611] In this study, Saccharomyces cerevisiae strain ST7574 (CEN.PK113-7D strain transformed with a plasmid carrying a Cas9 expression cassette and G418 resistance) was used as a background strain for metabolic engineering. Yarrowia lipolytica ST6512 (W29 strain with integrated Cas9 gene and D-serine resistance) was used as background strain for Yarrowia lipolytica engineering. Escherichia coli DH5α was used for all cloning procedures, propagation and storage of plasmids. Ergothioneine (catalogue #E7521-25MG, > 98% purity) was purchased from Sigma-Aldrich and histidine trimethyl inner salt (catalogue #H288900, 100 mg, > 95% purity) was purchased from Toronto Research Chemicals Inc. Synthetic genes were ordered through Thermo Fisher Scientific's GeneArt GeneSynthesis service or IDT's custom gene synthesis service. Sequencing results were obtained by Eurofins Genomics (Ebersbe...
Embodiment 2
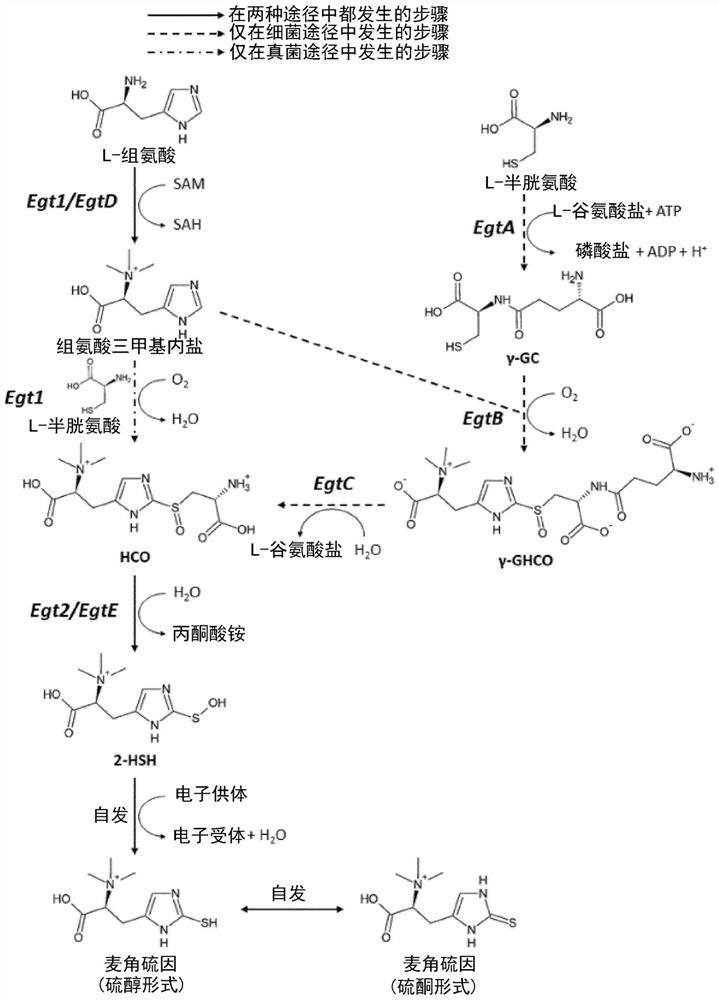
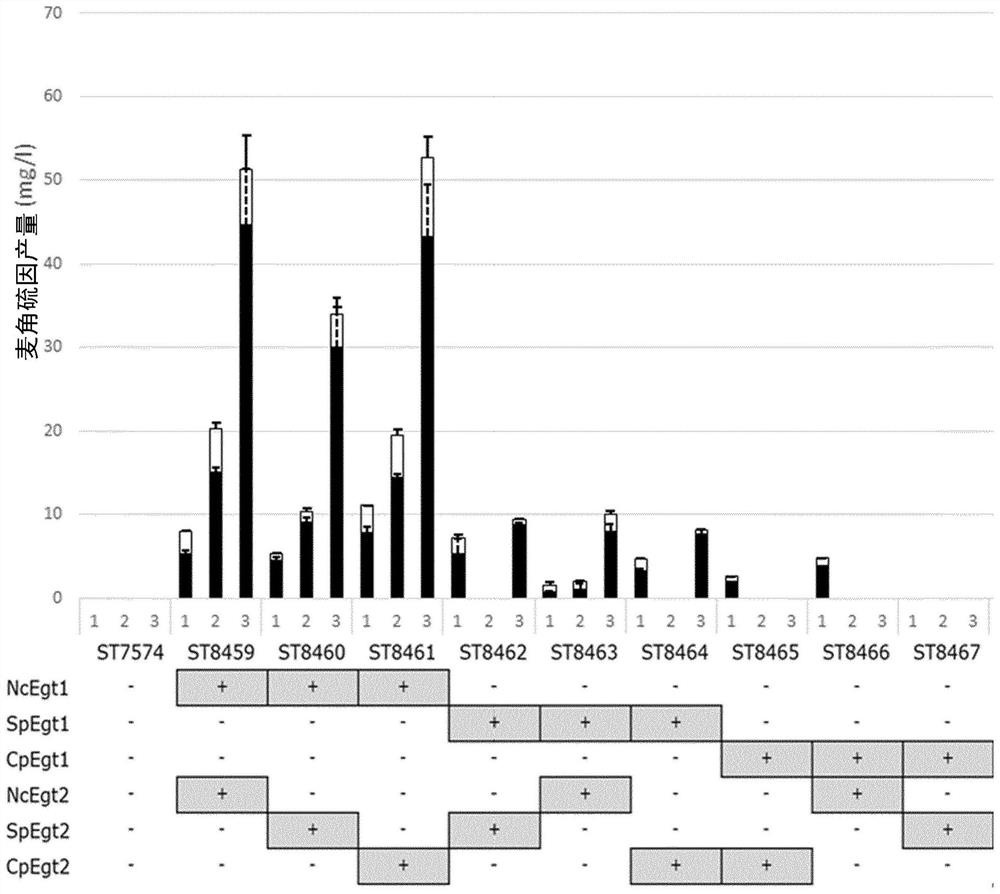
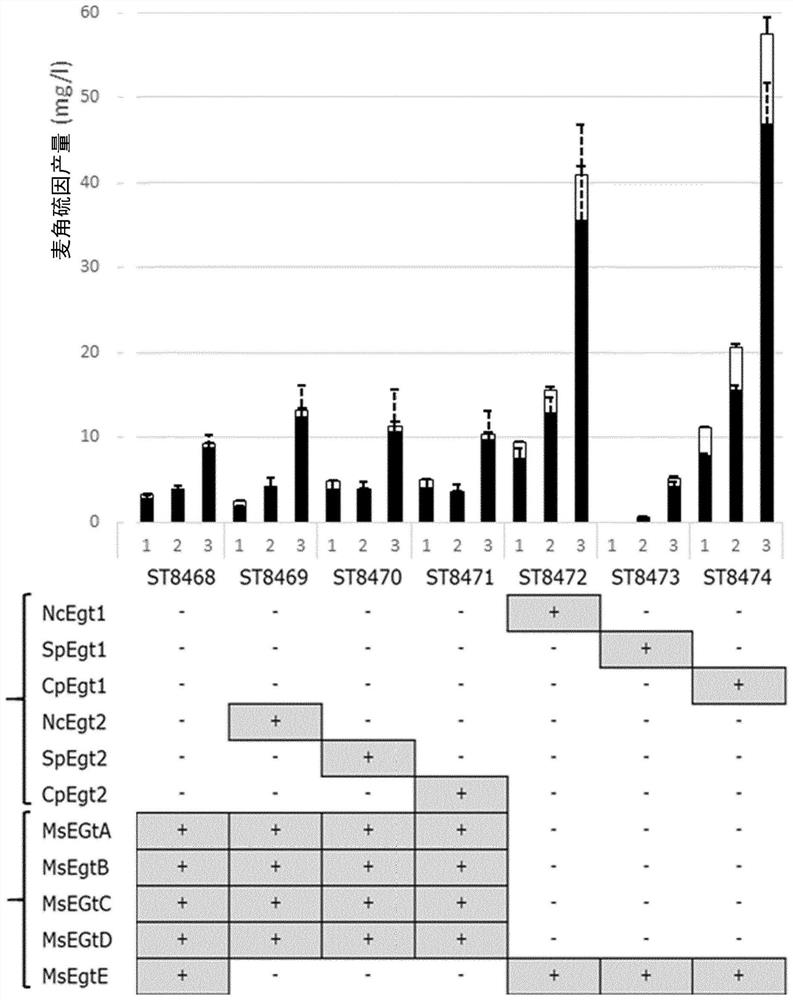
[0634] Example 2 - Results: Integration of the Ergothioneine Biosynthetic Pathway in Yeast
[0635] By using the Egt1 sequence of Neurospora crassa (Genbank accession number: XP_956324.3) in a BLAST search, we have identified Egt1 homologues in ergot and S. pombe (Genbank accession numbers: CCE33591.1 and NP_596639.2). Similarly, Egt2 homologues (Genbank accession numbers: XP_001728131.1 and CCE33140.1) were found in Neurospora crassa and Ergot sp. using Egt2 from Schizosaccharomyces pombe (Genbank accession number: NP_595091.1). The amino acid sequences of M. smegmatis genes EgtA, EgtB, EgtC, EgtD and EgtE were also taken from Genbank (Genbank accession numbers: AFP42520.1, WP_011731158.1, WP_011731157.1, WP_011731156.1, ABK70212.1). All genes were generated as synthetic DNA strings, codon-optimized for S. cerevisiae, except Egt1 and Egt2 from S. pombe, as they were amplified from genomic DNA extracts. A total of 16 pathway variants were assembled, of which 9 were fungal, 1...
Embodiment 3
[0637] Example 3 - Results: Ergothioneine Transporter
[0638] Since approximately half of the ERG produced remains in the cell, we investigated whether exporting ERG from yeast cells might at least partially limit production. The estimated wet weight concentration was 0.37mg / g wet weight yeast cells (taken from SC + 20g / l glucose + 1g / l His / Cys / Met), and the concentration of ERG inside the cells was 1.75mM, or 120 times higher than the culture medium. Since Mycobacterium smegmatis is known to secrete ergothioneine at levels up to 4 times the intracellular concentration, expressed in pg / 10 5 Given the CFU, we speculate that there must be an ERG transporter in its genome. Therefore, the biosynthetic ERG clusters in this organism were investigated. In addition to 5 known biosynthetic Egt genes, this cluster also contained 1 transmembrane protein, which we hypothesized might be an ERG transporter. To test the effect of the product of this gene on ERG production in yeast, the h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com