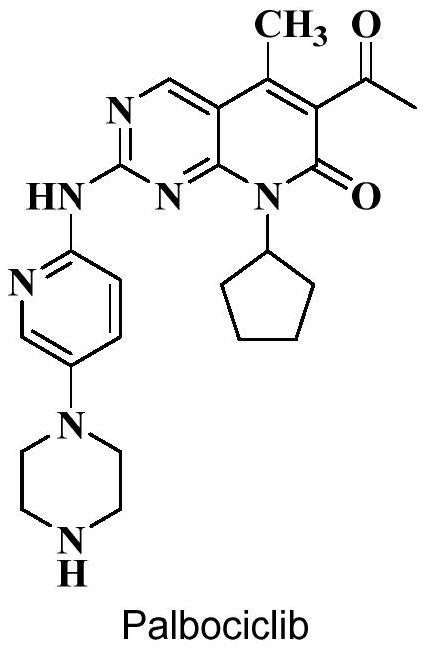
Preparation method of palbociclib intermediate
An intermediate and reaction time technology, which is applied in the field of preparation of Palbociclib intermediates, can solve the problems of many three wastes, many reaction steps, difficult process amplification, etc., and achieves the advantages of simple reaction system, short reaction time and shortened reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
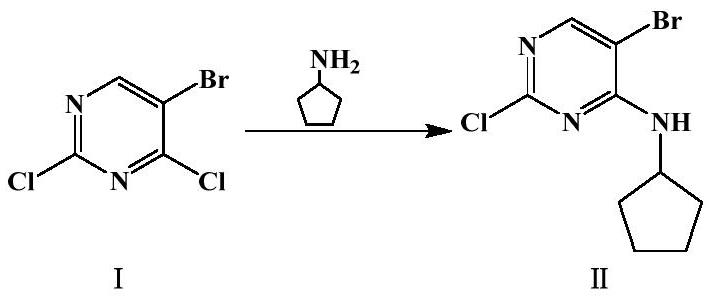
[0030] Preparation of 5-bromo-2-chloro-4-cyclopentylaminopyrimidine (Ⅱ)
[0031] The molar ratio of 5-bromo-2,4-dichloropyrimidine to cyclopentylamine is 1:1.2, and the mass ratio of 5-bromo-2,4-dichloropyrimidine to triethylamine is 1:3.1.
[0032] Take 5-bromo-2,4-dichloropyrimidine (7g, 0.03072mol) in a 100ml flask, add triethylamine (21.84g, 0.21583mol), and slowly add cyclopentylamine (3.14g, 0.03686mol), after the dropwise addition, react for 0.5h, TLC detects that the raw material has reacted completely, stop the reaction, remove triethylamine by rotary evaporation, add 30ml of water, stir for 1h and then filter with suction, wash the filter cake with 14ml of n-heptane, 50°C Dry under reduced pressure to obtain 7.66 g of white crystals, yield: 90.2%.
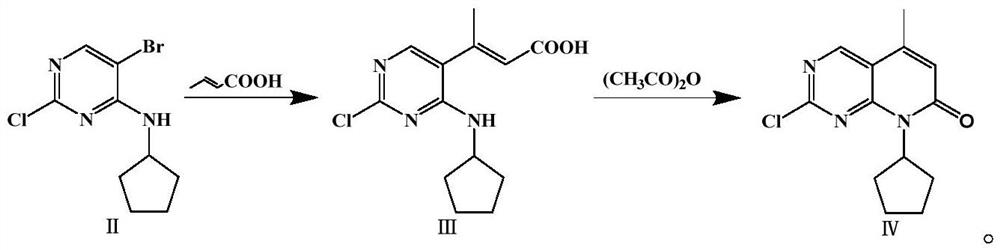
[0033] Preparation of 2-chloro-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Ⅳ)
[0034] The molar ratio of 5-bromo-2-chloro-4-cyclopentylaminopyrimidine to crotonic acid, diisopropylethylamine, palladium acet...
example 2
[0037] Preparation of 5-bromo-2-chloro-4-cyclopentylaminopyrimidine (Ⅱ)
[0038] The molar ratio of 5-bromo-2,4-dichloropyrimidine to cyclopentylamine is 1:1.15, and the mass ratio of 5-bromo-2,4-dichloropyrimidine to diisopropylethylamine is 1:3.1.
[0039] Take 5-bromo-2,4-dichloropyrimidine (5g, 0.0219mol) in a 100ml flask, add diisopropylethylamine (15.64g, 0.1210mol), and slowly add cyclopentylamine ( 2.15g, 0.0252mol), after the dropwise addition, react for 0.5h, TLC detects that the reaction of the raw materials is complete, stop the reaction, rotary evaporate most of the diisopropylethylamine, add 20ml of water, stir for 1h and then filter with suction, use 10ml of diisopropylethylamine for the filter cake Washed with n-heptane, dried under reduced pressure at 50°C to obtain 5.43 g of white crystals, yield: 89.5%.
[0040] Preparation of 2-chloro-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Ⅳ)
[0041] The molar ratio of 5-bromo-2-chloro-4-cyclopentylamino...
example 3
[0044] Preparation of 5-bromo-2-chloro-4-cyclopentylaminopyrimidine (Ⅱ)
[0045] The molar ratio of 5-bromo-2,4-dichloropyrimidine to cyclopentylamine is 1:1.2, and the mass ratio of 5-bromo-2,4-dichloropyrimidine to tripropylamine is 1:3.0.
[0046] Take 5-bromo-2,4-dichloropyrimidine (5g, 0.0219mol) in a 100ml flask, add tripropylamine (15.06g, 0.1051mol), and slowly add cyclopentylamine (2.24g, 0.02628 mol), after the dropwise addition, react for 0.5h, TLC detects that the reaction of the raw materials is complete, stop the reaction, distill off part of tripropylamine, add 30ml of water, stir for 1h and then filter with suction, wash the filter cake with 10ml of n-heptane, and dry under reduced pressure at 50°C , to obtain 5.29 g of white crystals, yield: 87.2%.
[0047] Preparation of 2-chloro-8-cyclopentyl-5-methyl-8H-pyrido[2,3-d]pyrimidin-7-one (Ⅳ)
[0048] The molar ratio of 5-bromo-2-chloro-4-cyclopentylaminopyrimidine to crotonic acid, diisopropylethylamine, pallad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com