Novel coronavirus nucleocapsid protein antibody or antigen binding fragment for in vitro diagnosis
A technology of nucleocapsid protein and coronavirus, which is applied in the direction of antiviral immunoglobulin, antibody, antiviral agent, etc., can solve the problems of low sensitivity, prone to missed detection, prone to false positive results, etc., to achieve high sensitivity and specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Antibodies were obtained by the hybridoma method. Specific steps are as follows:
[0066] The new coronavirus nucleocapsid protein was used as an antigen to immunize mice. Mouse spleens were collected and splenocytes were prepared. Mouse splenocytes were fused with Myeloma cells and stable hybridoma cells were screened. Antigen-specific antibodies were then screened by ELISA.
[0067] The obtained highly specific antibodies were sequenced. One of the antibody numbers is mAb#9, its heavy chain variable region sequence is shown in SEQ ID NO:17, and its light chain variable region sequence is shown in SEQ ID NO:18.
[0068] Then the coding sequence of the antibody was introduced into pCMV_HC and pCMV_LC antibody expression vectors. The pCMV_HC and pCMV_LC vectors were transfected into CHO cell lines by liposome method for expression of recombinant antibodies. The antibody was separated and purified with Protein A affinity column, and the purity reached more than 95%....
Embodiment 2
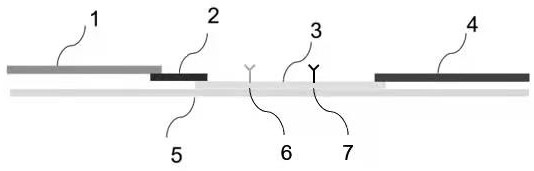
[0073] Embodiment 2 provides a kind of test strip, such as figure 1 shown. figure 1 The middle mark 1 is the sample pad (glass fiber film), 2 is the colloidal gold pad, 3 is the nitrocellulose membrane, 4 is the absorbent paper, 5 is the bottom plate, 6 is the detection line (also known as the T line), 7 is the quality Control line (also known as C line). The preparation method of each part in the test strip is as follows:
[0074] 1. Preparation of nitrocellulose membrane
[0075] Preparation of coating buffer: 0.05M pH8.5 PBS buffer is used as coating buffer, filtered through 0.22 μm membrane, and stored at 4°C for later use. The buffer formula: NaCl 40g, KCl 1g, NaCl 2 HPO 4 12H 2 O 14.5g, KH 2 PO 4 1g, dilute to 1000mL with double-distilled deionized water.
[0076] Preparation of nitrocellulose membrane: Dilute the recombinant antibody (mAb#4) of the new coronavirus nucleocapsid protein obtained in Example 1 to 0.5-2 mg / mL with coating buffer, adjust the machine...
Embodiment 3
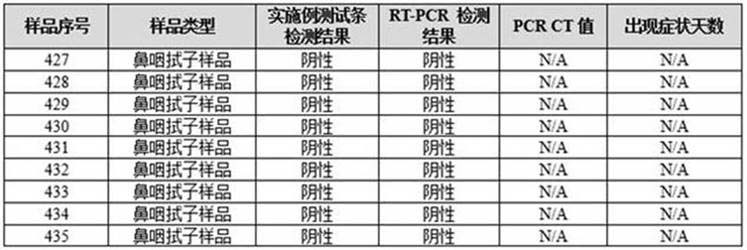
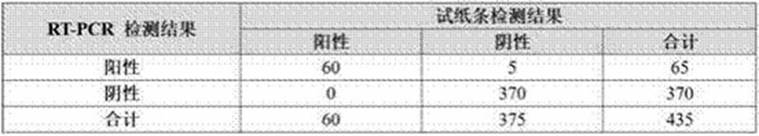
[0096] Embodiment 3 adopts the test strip of embodiment 2 to verify the detection performance of novel coronavirus:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com