Preparation method of roxadustat key intermediate
An intermediate, m-phenoxyacetophenone technology, applied in the preparation of ethyl 1-methyl-7-phenoxyisoquinoline-3-carboxylate, the key intermediate field of roxadustat, can solve Expensive and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
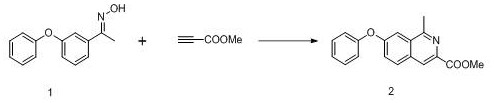
[0044] Add 2.41 g (0.01 mol) m-phenoxyacetophenone derivative 1, 0.15 g (2.5 mol%) dichloro(pentamethylcyclopentadienyl) Rhodium (III) dimer, 1.68 g (0.02 mol) of methyl propiolate, 1.64 g (0.02 mol) of sodium acetate, and 20 ml of tert-butanol were added, and the temperature was raised to reflux, and the reaction was continued for 24 h. After the TLC detection reaction was finished, the reaction solution was down to room temperature, 20 ml of water was added to the reaction solution, extracted 3 times with ethyl acetate, the organic phases were combined, washed with saturated sodium chloride and water successively, dried over anhydrous sodium sulfate, concentrated, The obtained concentrate was recrystallized from ethyl acetate and n-hexane, and dried to obtain 2.04 g of roxadustat intermediate 1-methyl-7-phenoxyisoquinoline-3-carboxylate, with a yield of 69.7%.
Embodiment 2
[0046]Add 2.41 g (0.01 mol) m-phenoxyacetophenone derivative 1, 0.15 g (2.5 mol%) dichloro(pentamethylcyclopentadienyl) Rhodium (III) dimer, 1.68 g (0.02 mol) of methyl propiolate, 1.96 g (0.02 mol) of potassium acetate, and 20 ml of tert-butanol were added, and the temperature was raised to reflux, and the reaction was continued for 24 hours. After the TLC detection reaction was finished, the reaction solution was down to room temperature, 20 ml of water was added to the reaction solution, extracted 3 times with ethyl acetate, the organic phases were combined, washed with saturated sodium chloride and water successively, dried over anhydrous sodium sulfate, concentrated, The obtained concentrate was recrystallized from ethyl acetate and n-hexane, and dried to obtain 2.09 g of roxadustat intermediate 1-methyl-7-phenoxyisoquinoline-3-carboxylic acid methyl ester, with a yield of 71.2%.
Embodiment 3
[0048] Add 2.41 g (0.01 mol) m-phenoxyacetophenone derivative 1, 0.15 g (2.5 mol%) dichloro(pentamethylcyclopentadienyl) Rhodium (III) dimer, 1.68 g (0.02 mol) of methyl propiolate, 3.34 g (0.02 mol) of silver acetate, and 20 ml of tert-butanol were added, and the temperature was raised to reflux, and the reaction was continued for 24 h. After the TLC detection reaction was finished, the reaction solution was down to room temperature, 20 ml of water was added to the reaction solution, extracted 3 times with ethyl acetate, the organic phases were combined, washed with saturated sodium chloride and water successively, dried over anhydrous sodium sulfate, concentrated, The obtained concentrate was recrystallized from ethyl acetate and n-hexane, and dried to obtain 2.44 g of roxadustat intermediate 1-methyl-7-phenoxyisoquinoline-3-carboxylic acid methyl ester, with a yield of 83.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com