Method for refining delafloxacin and intermediate thereof
A delafloxacin and body-style technology, which is applied in the field of refining delafloxacin and its intermediates, can solve the problems of being unsuitable for large-scale industrial production, and achieve the effects of reducing the generation of impurities, mild reaction temperature and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
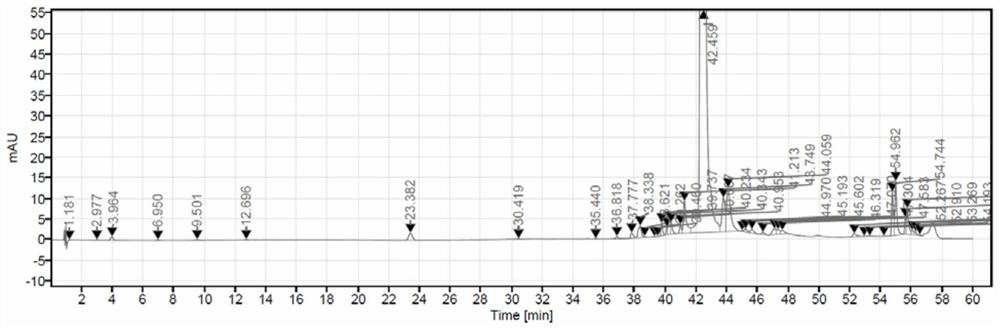
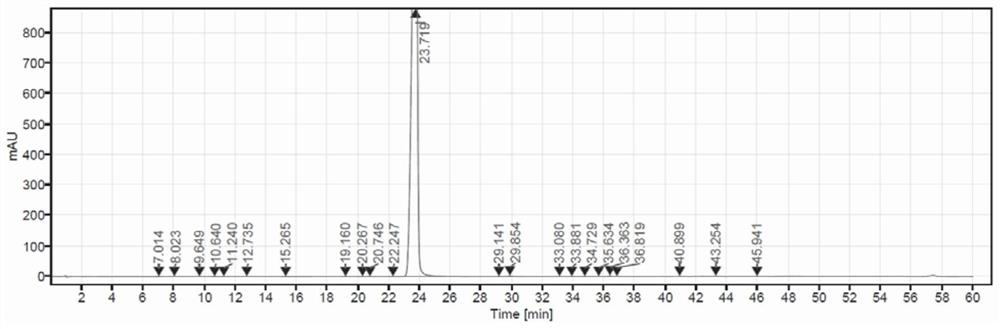
Image
Examples
Embodiment 1
[0041] Embodiment 1, the preparation of delafloxacin
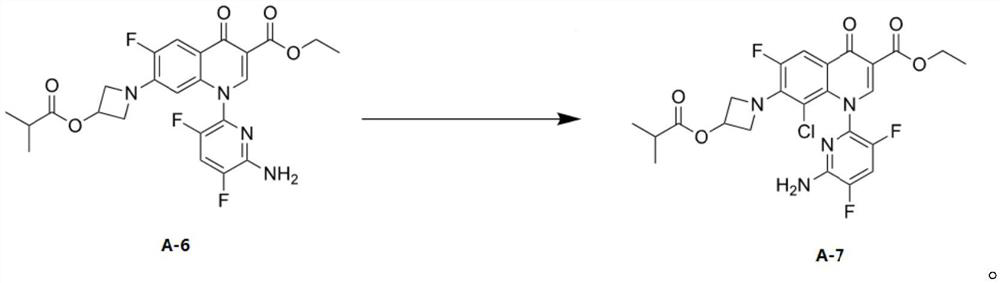
[0042] Add 50.0 g of the compound of formula A-6 and 150 ml of ethyl acetate into a 1 L reaction flask, and control the temperature at 10-35°C. Add NCS solution dropwise (completed by adding 15.6g NCS to the mixed solution of 0.13g sulfuric acid and 0.09g citric acid in 250ml methyl acetate), after the completion of the dropwise reaction for 6-10 hours, use 250g1.5% bicarbonate Sodium aqueous solution and 140 g of 10% sodium sulfite aqueous solution were washed and separated, and the solvent was evaporated in vacuo to obtain 51.0 g of the compound of formula A-7 with a yield of 95.7%. Add 30g formula A-7 compound, 240g isopropanol and potassium hydroxide aqueous solution (9.1g potassium hydroxide is dissolved in 225g water) in reaction bottle afterwards, after 3h of temperature control 10-35 ℃ react, add 12% acetic acid solution 143g , stirred for 1 h and then dried by suction to obtain 23.7 g of delafloxacin, with a yiel...
Embodiment 2
[0043] Embodiment 2, the preparation of delafloxacin
[0044]Add 50.0 g of the compound of formula A-6 and 150 ml of ethyl acetate into a 1 L reaction flask, and control the temperature at 10-35°C. Add NCS solution dropwise (completed by adding 15.6g NCS to the mixed solution of 0.04g sulfuric acid and 0.26g citric acid in 250ml methyl acetate), after the completion of the dropwise reaction for 6-10 hours, use 250g1.5% bicarbonate Sodium aqueous solution and 140 g of 10% sodium sulfite aqueous solution were washed and separated, and the solvent was evaporated in vacuo to obtain 49.6 g of the compound of formula A-7 with a yield of 93%. Add 30g formula A-7 compound, 240g isopropanol and potassium hydroxide aqueous solution (9.1g potassium hydroxide is dissolved in 225g water) in reaction bottle afterwards, after 3h of temperature control 10-35 ℃ react, add 12% acetic acid solution 143g , stirred for 1 h and then dried by suction to obtain 22.1 g of delafloxacin, with a yield o...
Embodiment 3
[0045] Embodiment 3, the preparation of delafloxacin
[0046] Add 50.0 g of the compound of formula A-6 and 150 ml of ethyl acetate into a 1 L reaction flask, and control the temperature at 10-35°C. Add dropwise NCS solution (by adding 15.6gNCS to the mixed solution of 250ml methyl acetate of 0.34g citric acid to complete), after the completion of the dropwise reaction for 6-10 hours, use 250g1.5% sodium bicarbonate aqueous solution, 140g10 % sodium sulfite aqueous solution was washed and separated, and the solvent was evaporated in vacuo to obtain 48.5 g of the compound of formula A-7, with a yield of 91.0%. Add 30g formula A-7 compound, 240g isopropanol and potassium hydroxide aqueous solution (9.1g potassium hydroxide is dissolved in 225g water) in reaction bottle afterwards, after 3h of temperature control 10-35 ℃ react, add 12% acetic acid solution 143g , stirred for 1 h and then dried by suction to obtain 20.0 g of delafloxacin with a yield of 81.1% and a purity of 92.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com