Orthopedic repair implant and preparation method thereof
An implant and orthopaedic technology, applied in the field of biomedical degradable material preparation, can solve the problems of poor mechanical and mechanical properties of pure zinc implants and can not meet functional requirements, and achieves elimination of homogenization and segregation within grains, increase in surface area, The effect of accelerating the degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] In the embodiment of the present invention, the preparation method of biomedical degradable zinc alloy sheet material and film material from metal raw materials specifically includes:
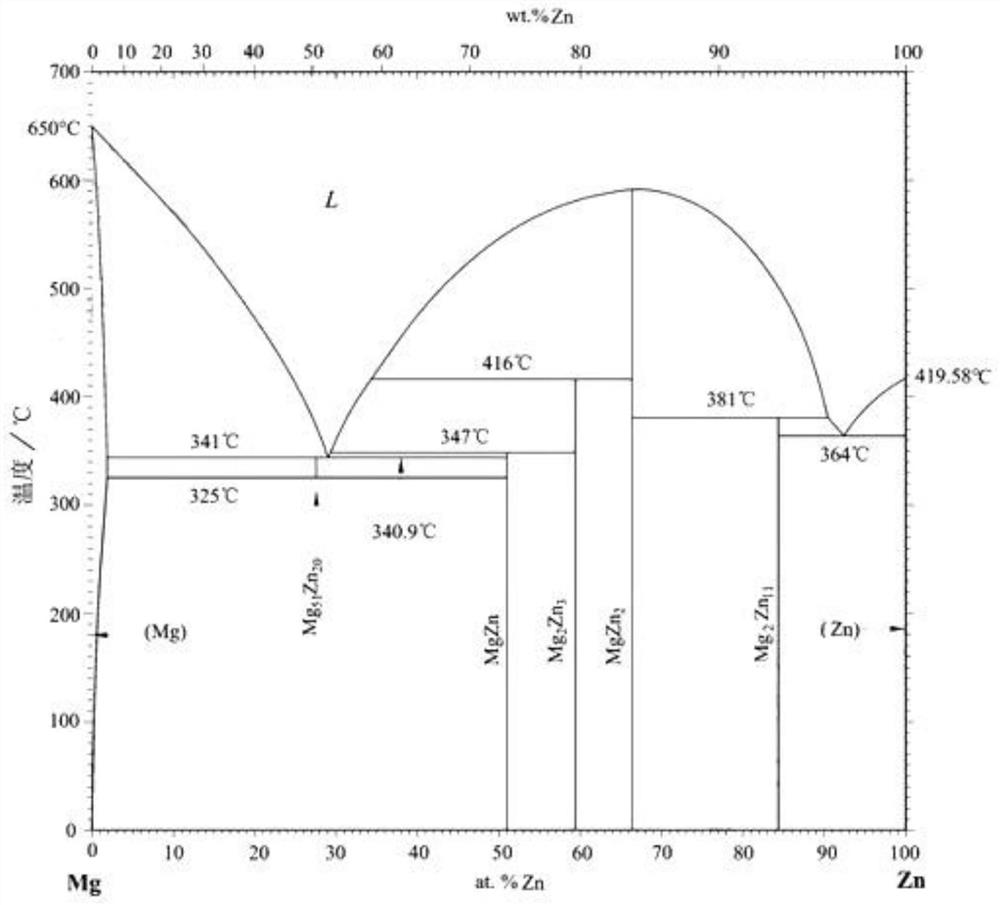
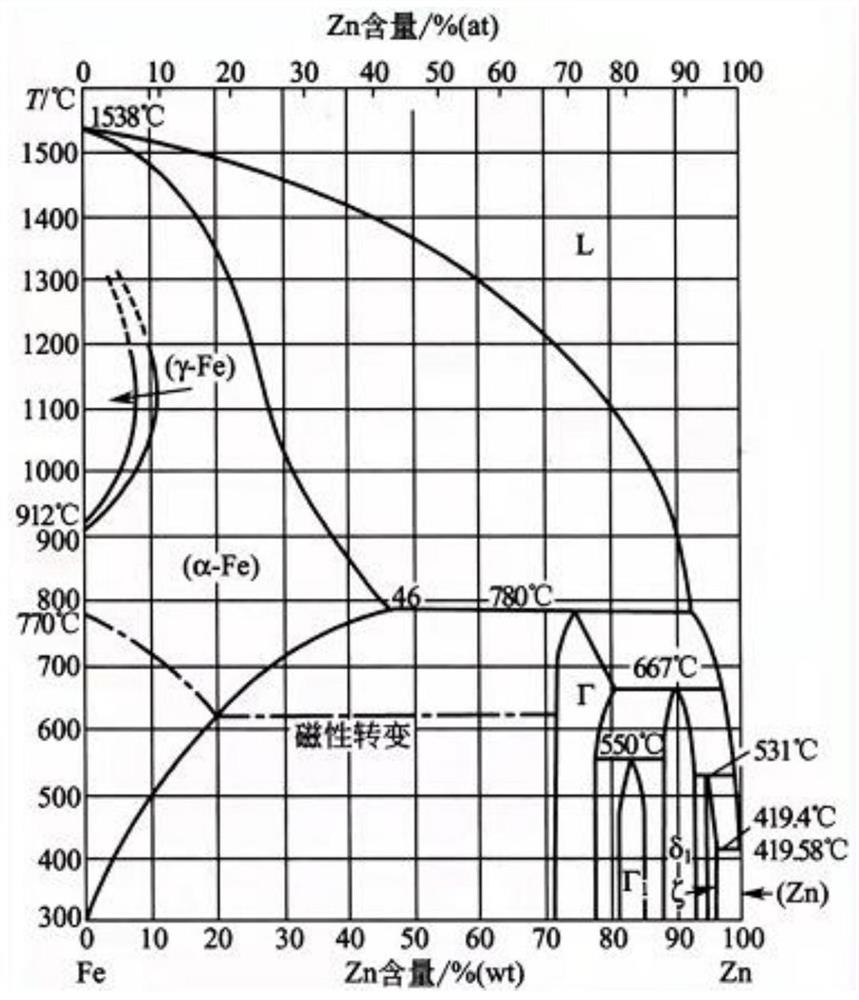
[0038] Melting: smelting magnesium-zinc master alloy α1, iron-zinc master alloy β1, and solid solution master alloy γ1 of functional elements and zinc elements respectively. Then, the magnesium-zinc master alloy α1, the iron-zinc master alloy β1, the solid solution master alloy γ1 of functional elements and zinc elements, and the zinc ingot are smelted at a certain ratio, and the smelting solution is cast at 350-800°C in a cylindrical or In the cubic inner cavity, it is gradually cooled to room temperature to form a casting blank; the melting furnace needs to use an intermediate frequency / high frequency vacuum smelting furnace. The magnesium-zinc master alloy α1 contains at least Mg 2Zn 11 , MgZn 2 One of two co-crystals. The β1 iron-zinc master alloy contains at least FeZn 7 , FeZn...
Embodiment 1
[0064] This embodiment designs biomedical degradable zinc alloy bone plate
[0065] Formula: the biomedical degradable zinc alloy is Zn-Fe-Mg-Sn. Among them, the mass percentage content of Fe element is: 0.1%, the mass percentage content of Mg element is: 0.1%, the mass percentage content of Sn element is 0.0005%; the unavoidable impurities, the mass percentage content of a single element is not more than 0.005% , the total amount is not more than 0.01%, and the zinc element is the balance.
[0066] Preparation:
[0067] Melting: smelting magnesium-zinc master alloy α1-Zn20Mg respectively (the smelting mass ratio of metal raw materials Mg and Zn is 20:80, after mixing and melting at 580°C, casting the melt into an intermediate mold with a mold temperature of 381°C, With natural cooling to room temperature) and iron-zinc intermediate alloy β1-Zn10Fe (the melting mass ratio of metal raw materials Fe and Zn is 10:90, after mixing and melting at 672°C, cast the melt into an inte...
Embodiment 2
[0079] This embodiment relates to a biomedical degradable zinc alloy barrier repair film
[0080] Formula: the biomedical degradable zinc alloy is Zn-Fe-Mg-Sn. Among them, the mass percentage content of Fe element is: 0.2%, the mass percentage content of Mg element is: 0.04%, the mass percentage content of Sn element is: 0.0008%; for unavoidable impurities, the mass percentage content of a single element is not more than 0.005%, the total amount is not more than 0.01%, zinc element is the balance.
[0081] Preparation:
[0082] Melting: smelting magnesium-zinc master alloy α1-Zn2Mg respectively (the smelting mass ratio of metal raw materials Mg and Zn is 2:98, after mixing and melting at 580°C, casting the melt into an intermediate mold with a mold temperature of 364°C, With natural cooling to room temperature) and iron-zinc intermediate alloy β1-Zn8Fe (the melting mass ratio of metal raw materials Fe and Zn is 8:92, after mixing and melting at 672 ° C, the melt is cast to a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com