Sorafenib tosylate content determination method based on nuclear magnetic resonance quantitative technology
A technology of toluenesulfonic acid and sorafenib, which is applied in the field of determination of sorafenib toluenesulfonate content based on NMR quantitative technology, can solve the problems of complicated and cumbersome experimental process, and achieve simple sample processing, time-saving and exclusive The effect of high sex and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
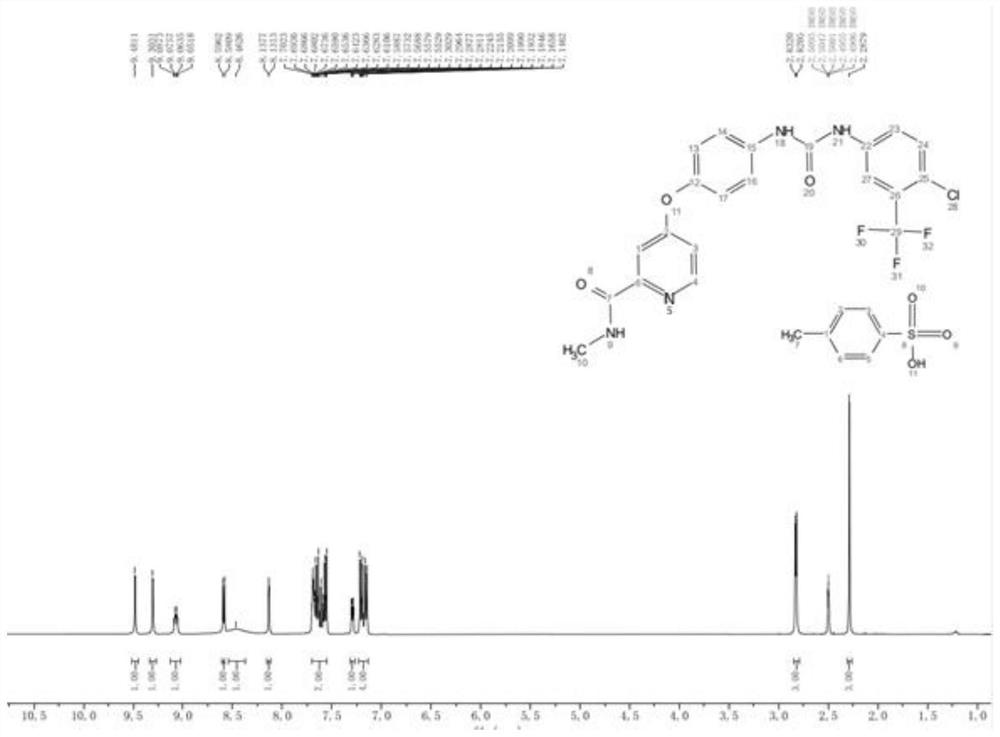
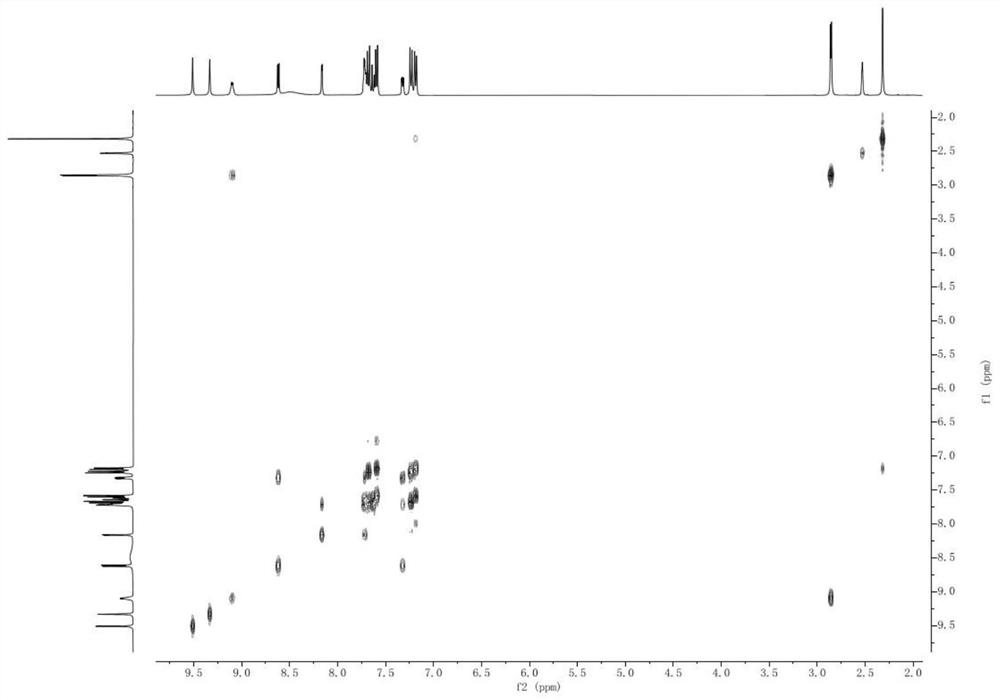
[0028] A kind of Sorafenib tosylate content assay method based on nuclear magnetic resonance quantitative technology, comprises the following steps:
[0029] S1. Preparation of internal standard solution: Accurately weigh 55.2 mg of hydroquinone into a 10 mL volumetric flask, constant volume with deuterated dimethyl sulfoxide, and record the obtained solution as hydroquinone internal standard solution;
[0030] S2, need testing solution: weigh the sorafenib tosylate crude drug and add 0.6mL hydroquinone internal standard solution and fully mix, the obtained sorafenib tosylate solution concentration is not higher than 20.0mg / ml, The resulting solution is then transferred to an NMR tube to be tested;
[0031] S3, nuclear magnetic resonance spectrum determination: adopt hydrogen nuclear magnetic resonance spectrum quantitative method, with the deuterated dimethyl sulfoxide in the obtained sorafenib tosylate solution in step 2 as solvent, hydroquinone as internal standard for quan...
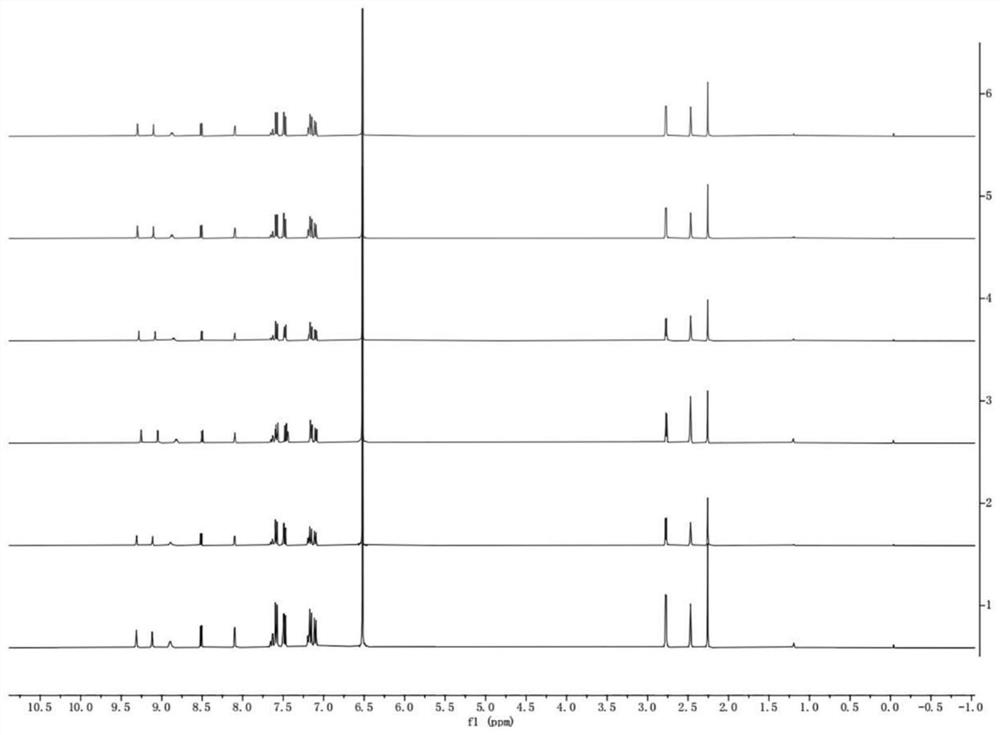
Embodiment 2
[0033] The difference between this embodiment and embodiment 1 is:
[0034] The relaxation delay time (d1) is 1s, and the sampling time (aq) is 10min.
Embodiment 3
[0036] The difference between this embodiment and embodiment 1 is:
[0037] The relaxation delay time (d1) is 5s, and the sampling time (aq) is 16min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com