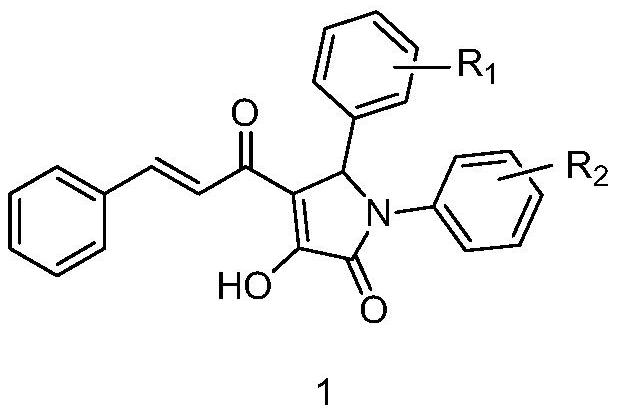
Application of 4-cinnamyl-3-hydroxyl pyrrolidone compound in preparation of medicine for treating diabetes mellitus
The technology of a diabetes drug and compound is applied in the application field of 4-cinnamyl-3-hydroxypyrrolidone compound, preparation and treatment of diabetes drug, can solve problems such as inability to completely cure, and achieves improvement of oral glucose tolerance and insulin sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
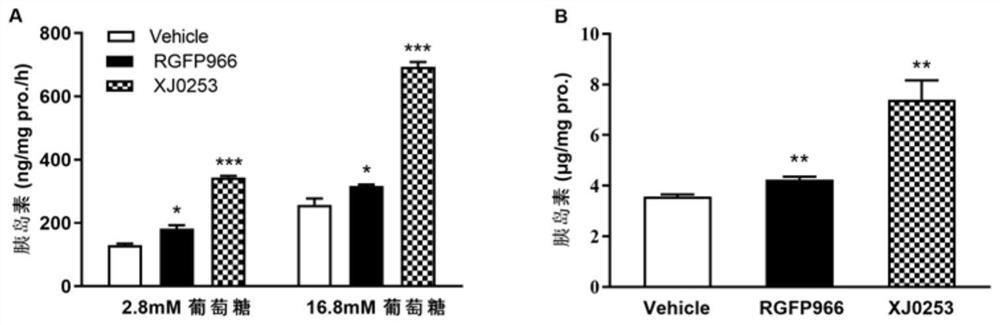
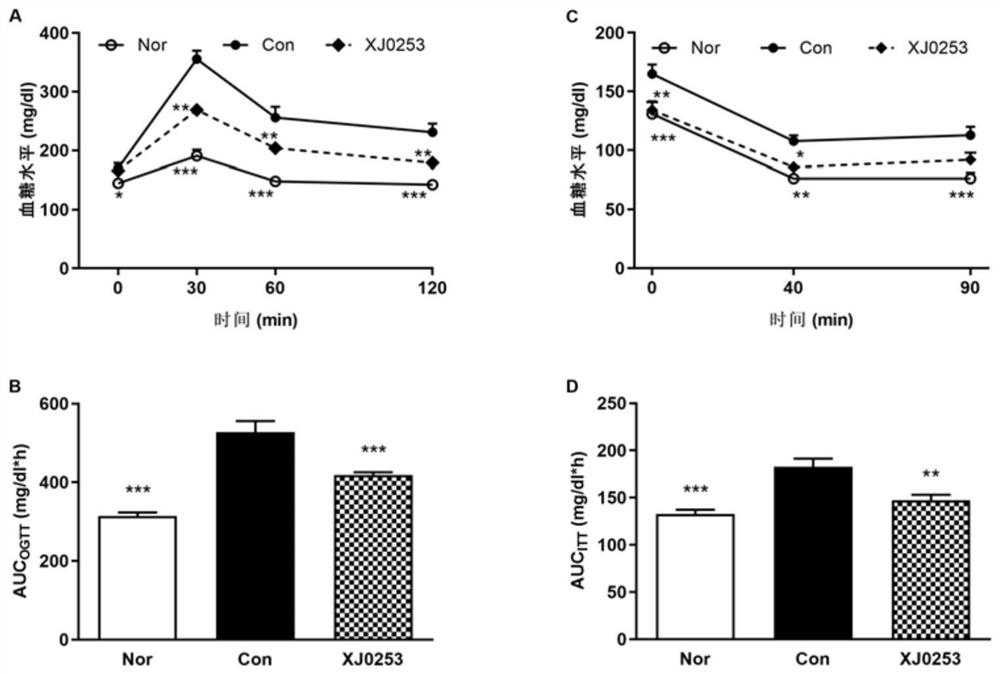
Image
Examples
Embodiment 1
[0058] Example 1. Acquisition of compounds XJ0240-XJ0245
[0059] The compound can be purchased from Compound Handling B.V. (Trade name: Specs), whose company address is Bleiswijkseweg 55, 2712PB Zoetermeer, The Netherlands, online shopping address: https: / / www.specs.net / .
[0060]
[0061]
Embodiment 2
[0062] Example 2. The acquisition of compound XJ2046-XJ0273
[0063] Compounds are commercially available from ChemDiv Inc., 12760 High Bluff Drive, Suite 370 San Diego, CA 92130 USA. Online shopping address: https: / / www.chemdiv.com / .
[0064]
[0065]
[0066]
[0067]
[0068] 2. Evaluation of the biological activity of the compound
experiment example 1
[0069] Experimental example 1. Determination of HDAC3 / NCoR1 enzyme inhibitory activity
[0070] 1. Experimental method
[0071] Enzyme inhibitory activity was determined by Enzo's HDAC3 / NCoR1 Fluorometric Drug Discovery Kit. Reactions were performed in 96-well plates. Dilute the compound to 5×C with Assay Buffer II Final The sample buffer and the vehicle DMSO were added to Buffer II in the same volume as a negative control. Add 25 μL Assay Buffer II (blank control), or 10 μL negative control, or sample buffer (positive control TSA, or test compound) to each well; dilute the enzyme recombinant complex HDAC3 / NCoR1 to 3.3×C with Assay Buffer II Final Enzyme working solution, added to each well (15μL / well, except blank control); Dilute the substrate FLUOR DE with Assay Buffer -SIRT1 to 2×C Final Substrate working solution, and preheated to 37°C, add 25 μL of preheated substrate working solution to all wells, start the reaction after mixing well, incubate at 37°C for 15-20 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com