Preparation method of methyl 2, 3-diaminobenzoate
A technology of methyl diaminobenzoate and nitrobenzoic acid, which is applied in the field of preparation of methyl 2,3-diaminobenzoate, can solve problems such as low purity, high raw material prices, and risk of explosion, and achieve a synthetic route Short, simple preparation method, easy to handle and purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
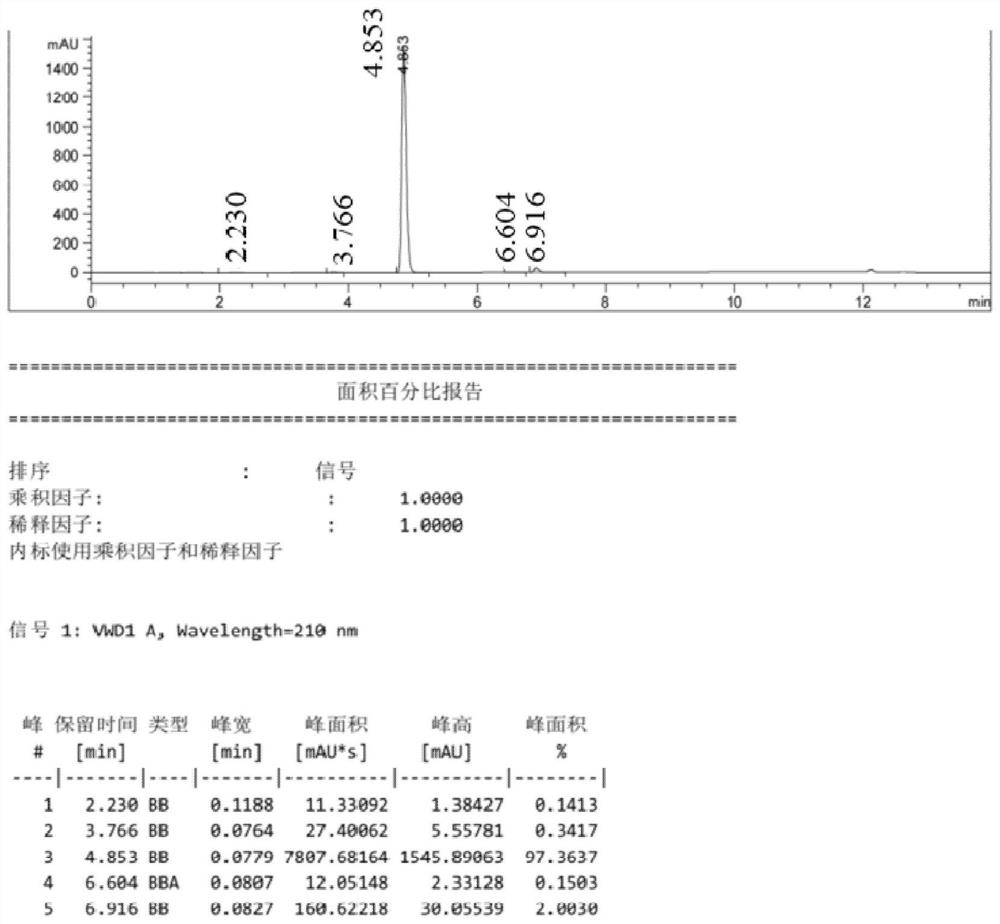
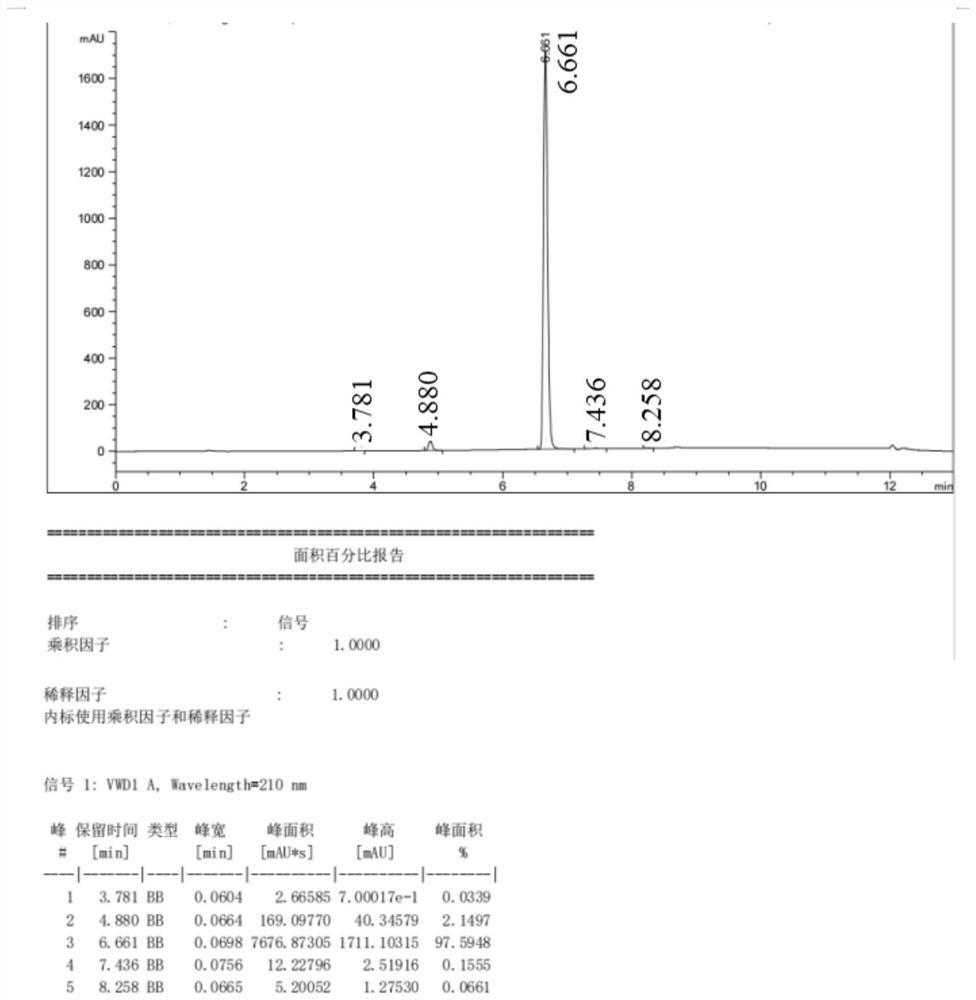
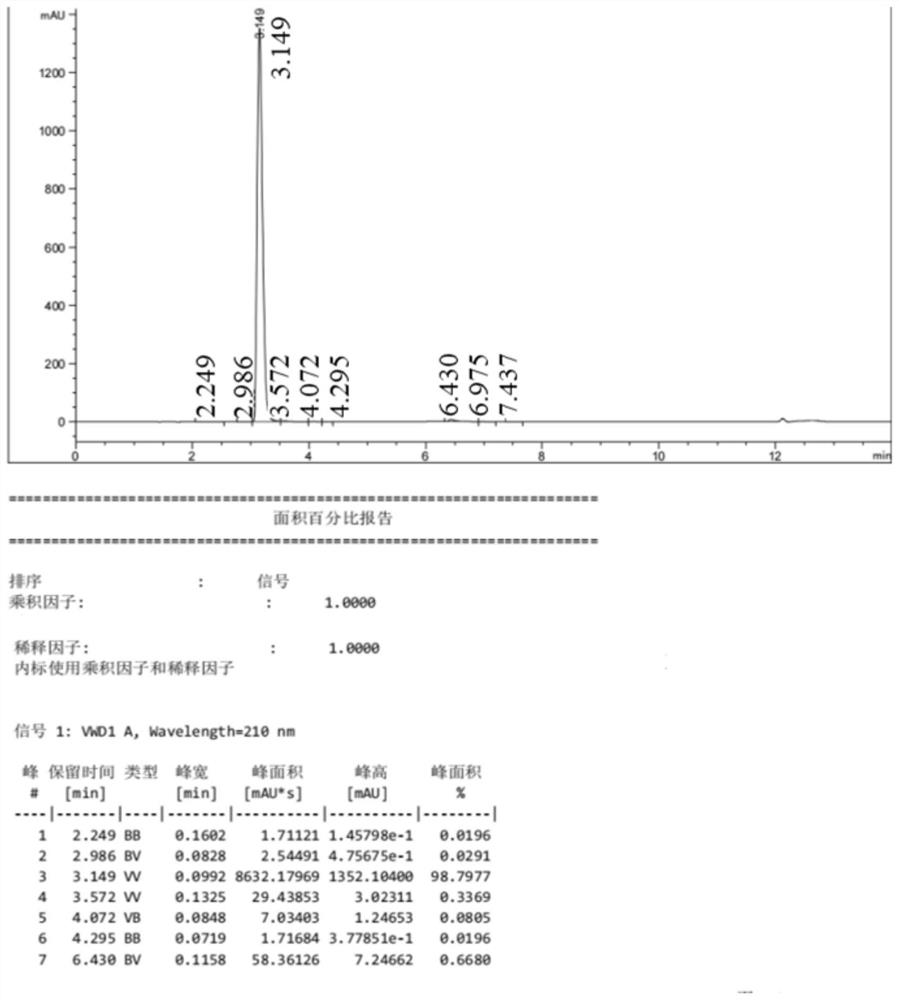
Image
Examples
Embodiment 1
[0036]
[0037] (1) Synthesis of 3-amino-2-nitrobenzoic acid (2):
[0038] Add 150mL of purified water and 0.83g (0.006mol) of copper chloride to a 250mL four-necked bottle, then add 25g (0.124mol) of 3-chloro-2-nitrobenzoic acid, stir, and feed ammonia gas (flow rate 0.1ml Every minute), be warming up to 120 ℃, insulation reaction 6 hours, treat that reaction finishes, close ammonia gas, cool down, crystallize, filter, dry to obtain 21.3g of 3-amino-2-nitrobenzoic acid, purity is 97.4%, yield The rate is 92.8%.
[0039] (2) Synthesis of 3-amino-2-nitrobenzoic acid methyl ester (3):
[0040] Add 50.0 g (0.273 mol) of 3-amino-2-nitrobenzoic acid and 800 mL of methanol into a 1000 mL four-neck flask, resulting in a yellow turbid system. Control the temperature at 20-30°C, add 40.1g (0.410mol) of concentrated sulfuric acid, raise the temperature to 70-75°C, stir for 4 hours, then cool down to below 5°C. Control the temperature T≤5°C, add sodium hydroxide to adjust the pH of...
Embodiment 2
[0044]
[0045] (1) Synthesis of 3-amino-2-nitrobenzoic acid (2):
[0046] Add 150mL of purified water and 16.7g (0.124mol) of copper chloride to a 250mL four-necked bottle, then add 25g (0.124mol) of 3-chloro-2-nitrobenzoic acid, stir, and feed ammonia gas (flow rate 0.1ml Every minute), be warming up to 120 ℃, insulation reaction 6 hours, treat that reaction finishes, close ammonia gas, cool down, crystallize, filter, dry to obtain 21.3g of 3-amino-2-nitrobenzoic acid, purity is 98.2%, yield The rate is 92.6%.
[0047] (2) Synthesis of 3-amino-2-nitrobenzoic acid methyl ester (3):
[0048]Add 50.0 g (0.273 mol) of 3-amino-2-nitrobenzoic acid and 800 mL of methanol into a 1000 mL four-neck flask, resulting in a yellow turbid system. Control the temperature at 20-30°C, add 40.3g (0.411mol) of concentrated sulfuric acid, raise the temperature to 70-75°C, stir for 4 hours, then cool down to below 5°C. Control the temperature T≤5°C, add sodium hydroxide to adjust the pH of ...
Embodiment 3
[0052]
[0053] (1) Synthesis of 3-amino-2-nitrobenzoic acid (2):
[0054] Add 150mL of purified water and 16.7g (0.124mol) of copper chloride to a 250mL four-necked bottle, then add 25g (0.124mol) of 3-chloro-2-nitrobenzoic acid, stir, and feed ammonia gas (flow rate 0.1ml Every minute), be warming up to 120 ℃, insulation reaction 6 hours, treat that reaction finishes, close ammonia gas, cool down, crystallize, filter, dry to obtain 21.3g of 3-amino-2-nitrobenzoic acid, purity is 97.2%, yield The rate is 91.6%.
[0055] (2) Synthesis of 3-amino-2-nitrobenzoic acid methyl ester (3):
[0056] Add 50.0 g (0.273 mol) of 3-amino-2-nitrobenzoic acid and 800 mL of methanol into a 1000 mL four-neck flask, resulting in a yellow turbid system. Control the temperature at 20-30°C, add 53.5g (0.546mol) of concentrated sulfuric acid, raise the temperature to 70-75°C, stir for 4 hours, then cool down to below 5°C. Control the temperature T≤5°C, add sodium hydroxide to adjust the pH of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com