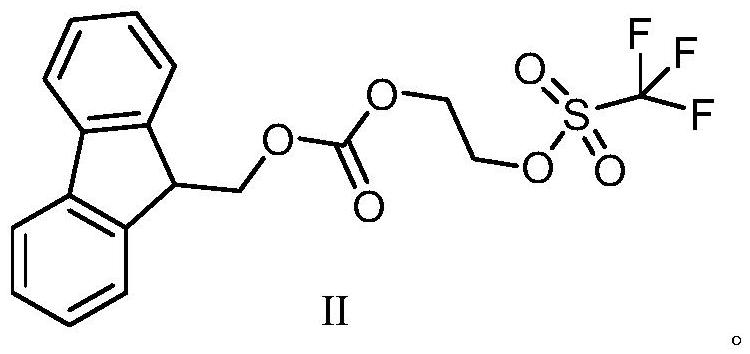
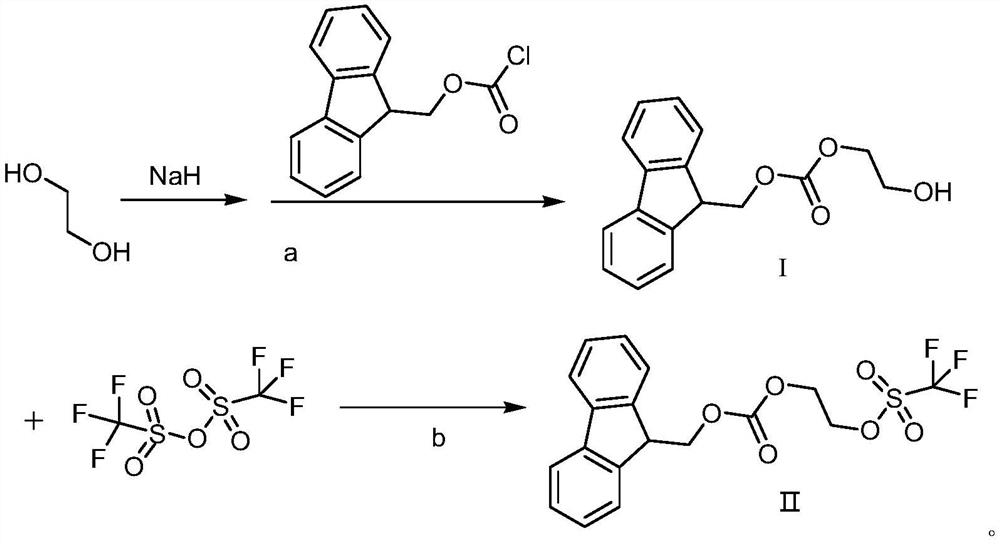
Derivative compound of ethylene glycol
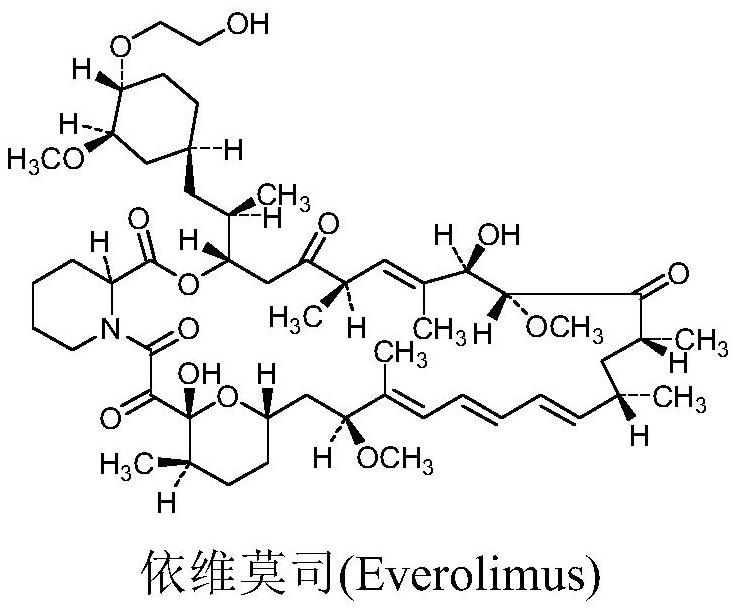
A compound and ethylene glycol technology, applied in the application field of the preparation of everolimus, can solve the problems of low yield of the target product, many by-products, poor stability, etc., achieve a reliable process route, less impurities, reduce degradation and The effect of impurity generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Add 62.1g of ethylene glycol and 370ml of tetrahydrofuran into the round-bottomed flask, stir and cool down to -5~5°C, add 40g of sodium hydride in batches, after the addition is complete, slowly rise to room temperature, and continue the reaction until the reaction is complete (monitored by TLC) , cooled to 5 ~ 10 ° C, under stirring, dropwise added 850ml of Fmoc-Cl (284.57g) tetrahydrofuran solution, after the dropwise addition was completed, it was raised to room temperature, and the reaction was continued until the reaction was complete (monitored by TLC); filtered, the filtrate was collected, evaporated under reduced pressure Remove the solvent, add 900ml ethyl acetate and 500ml purified water to the residue, extract and separate the liquid, wash with 300ml saturated brine, add an appropriate amount of anhydrous sodium sulfate to dry, evaporate the solvent under reduced pressure, rectify under reduced pressure, and collect the vacuum The fraction not lower than -0.09...
Embodiment 2
[0044] In the round bottom flask, add 62.1g of ethylene glycol and 310ml of tetrahydrofuran, stir to cool down to -5~5°C, add 36g of sodium hydride in batches, after the addition is completed, slowly rise to room temperature, and continue the reaction. After the reaction is complete (monitored by TLC ), cooled to 5-10°C, under stirring, added dropwise 850ml of Fmoc-Cl (271.6g) tetrahydrofuran solution, after the dropwise addition was completed, it was raised to room temperature, and the reaction was continued until the reaction was complete (monitored by TLC); filtered, and the filtrate was collected under reduced pressure Evaporate the solvent, add 900ml ethyl acetate and 500ml purified water to the residue, extract and separate the liquid, wash with 300ml saturated brine, add an appropriate amount of anhydrous sodium sulfate to dry, evaporate the solvent under reduced pressure, rectify under reduced pressure, collect the vacuum The fraction 191.8g with a temperature not lower...
Embodiment 3
[0047] In the round bottom flask, add 62.1g ethylene glycol and 420ml tetrahydrofuran, stir and cool down to -5~5°C, add 43.8g sodium hydride in batches, after the addition is completed, slowly rise to room temperature, and continue the reaction. After the reaction is complete (TLC monitoring), cooled to 5-10°C, and under stirring, added dropwise 900ml of Fmoc-Cl (310.4g) tetrahydrofuran solution, after the dropwise addition, rose to room temperature and continued to react until the reaction was complete (monitored by TLC); filtered, collected the filtrate, and reduced Remove the solvent by pressure evaporation, add 900ml ethyl acetate and 500ml purified water to the residue, extract and separate the liquid, wash with 300ml saturated brine, add an appropriate amount of anhydrous sodium sulfate to dry, evaporate the solvent under reduced pressure, rectify under reduced pressure, and collect The fraction 188.1g with a vacuum degree not lower than -0.095MPa and a fraction temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com