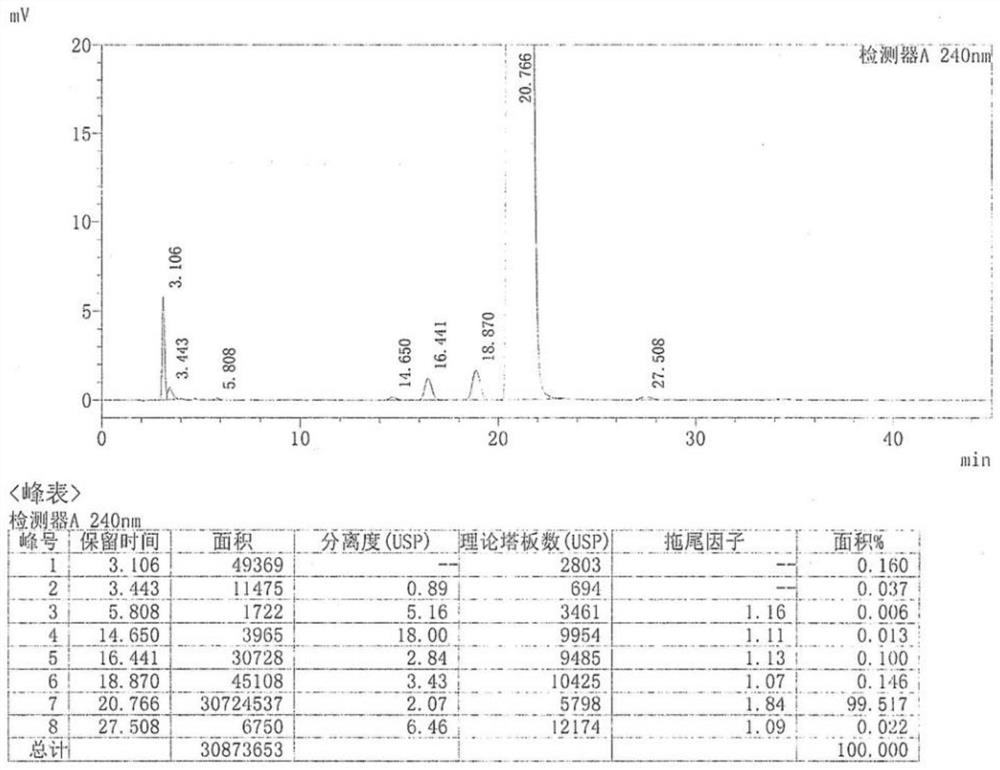
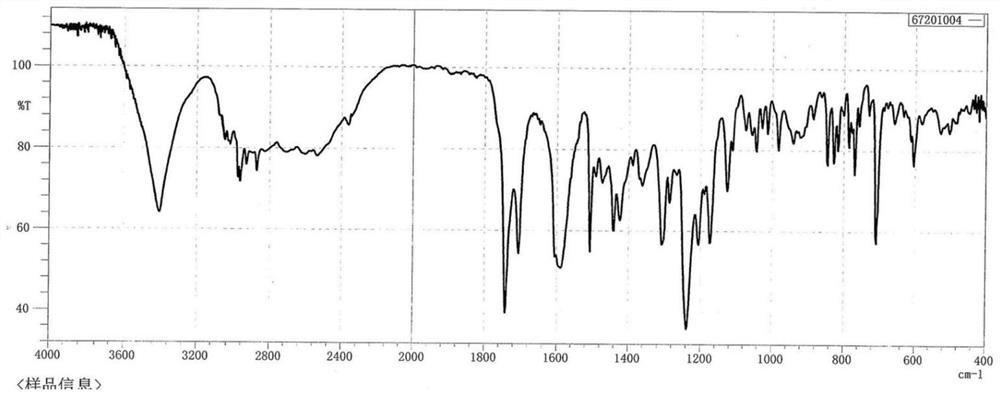
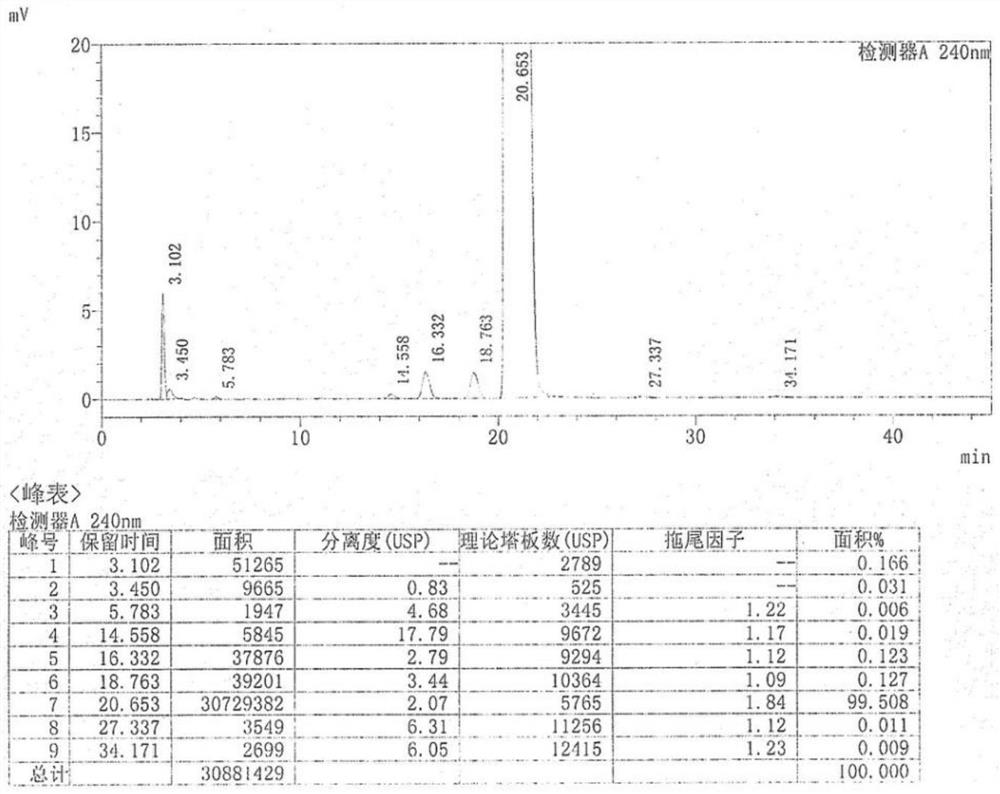
Industrial preparation process of tamoxifen citrate
A tamoxifen and preparation technology, which is applied in the field of drug synthesis, can solve the problems of restricting the industrial production of tamoxifen citrate, difficulty in product purification, and high cost, and achieve easy recovery and application, easy control of process conditions, The effect of low unit consumption of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Etherification:
[0061] Add 250.0kg of toluene to the etherification kettle, start stirring, add 23.00kg of sodium hydroxide and 25.00kg of p-bromophenol, raise the temperature to 112°C, reflux with water until no water drops appear continuously, and lower the inner temperature of the etherification kettle to 60°C Next, add 25.00kg of dimethylaminoethyl chloride hydrochloride, raise the temperature to 112°C, reflux reaction until no water droplets appear continuously in the water separator and the reflux toluene is clear, and the reaction is completed; wait until the inner temperature of the etherification tank drops to 35°C Next, put the feed liquid into a centrifuge to filter, rinse the filter residue with toluene, pump the filtrate and eluent into the toluene still, add purified water to stir, let it stand, and separate the water layer, then depressurize at -0.060MPa and 80°C Distillation, the distilled toluene is recovered and used mechanically, and finally the...
Embodiment 2
[0069] (1) Etherification:
[0070] Add 250.0kg of toluene to the etherification kettle, start stirring, add 21.00kg of sodium hydroxide and 25.00kg of p-bromophenol, raise the temperature to 110°C, reflux with water until no water drops appear continuously, and lower the inner temperature of the etherification kettle to 60°C Next, add 23.00kg of dimethylaminoethyl chloride hydrochloride, raise the temperature to 110°C, reflux reaction until no water drops appear continuously in the water separator and the reflux toluene is clear, and the reaction is completed; wait until the inner temperature of the etherification tank drops to 30°C Next, put the feed liquid into a centrifuge for filtration, rinse the filter residue with toluene, pump the filtrate and eluent into the toluene still, stir for 20 minutes, and separate the water layer after standing still, then carry out at -0.070MPa, 70°C temperature Distilled under reduced pressure, the distilled toluene was recovered and used ...
Embodiment 3
[0078] (1) Etherification:
[0079]Add 250.0kg of toluene to the etherification kettle, start stirring, add 25.00kg of sodium hydroxide and 25.00kg of p-bromophenol, raise the temperature to 112°C, reflux with water until no water drops appear continuously, and lower the inner temperature of the etherification kettle to 60°C Next, add 27.00kg of dimethylaminoethyl chloride hydrochloride, raise the temperature to 112°C, reflux reaction until no water drops appear continuously in the water separator and the reflux toluene is clear, the reaction is completed; wait until the inner temperature of the etherification tank drops to 30°C Next, put the feed liquid into a centrifuge for filtration, rinse the filter residue with toluene, pump the filtrate and eluent into the toluene distillation kettle, stir for 20 minutes, separate the water layer after standing, and then carry out at -0.090MPa, 70°C temperature Distilled under reduced pressure, the distilled toluene was recovered and us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com