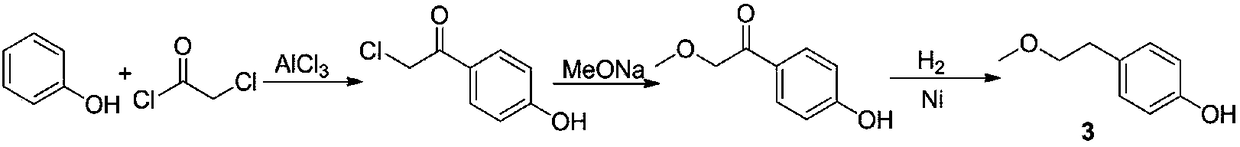
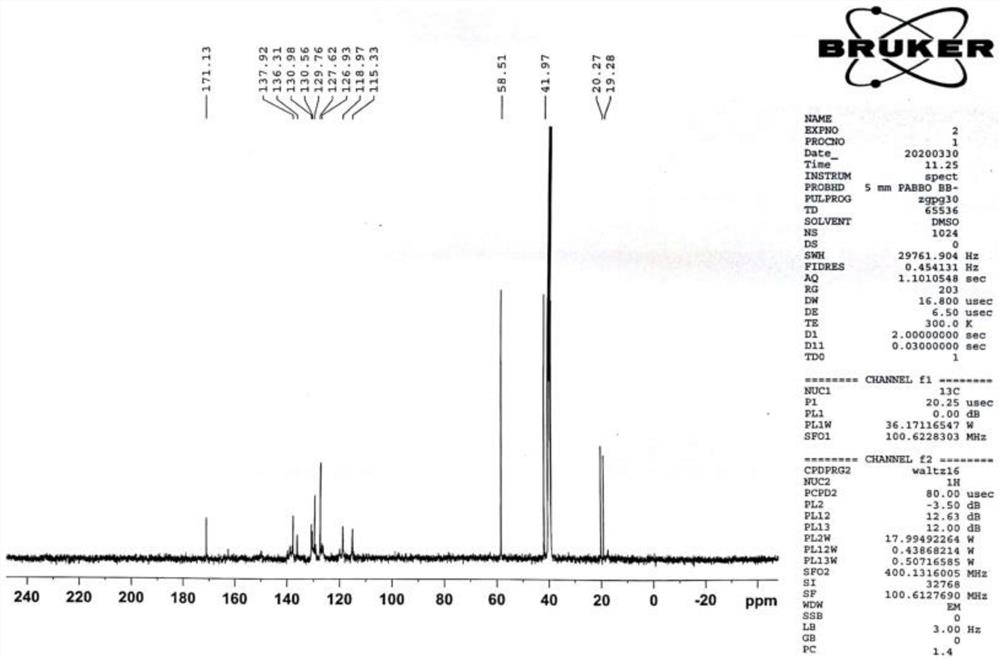
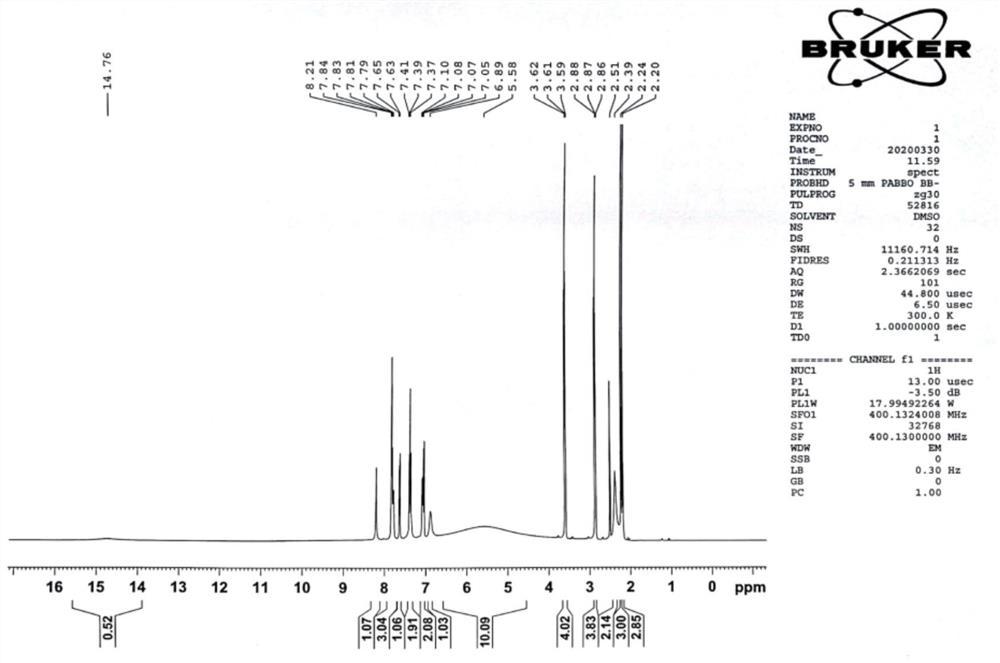
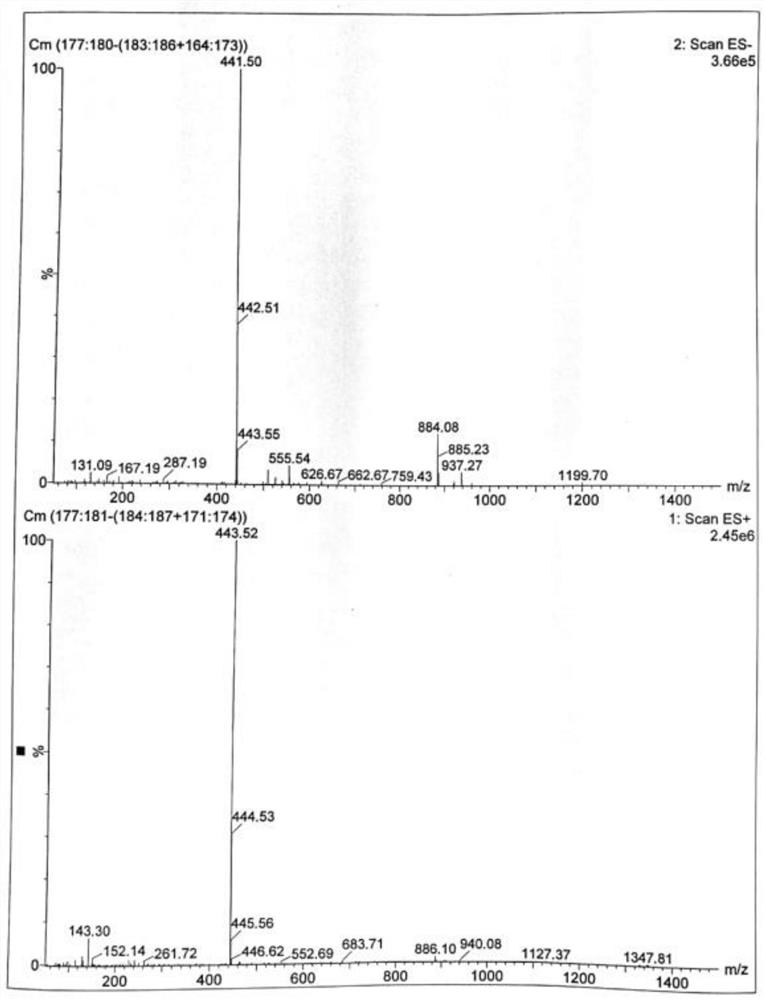
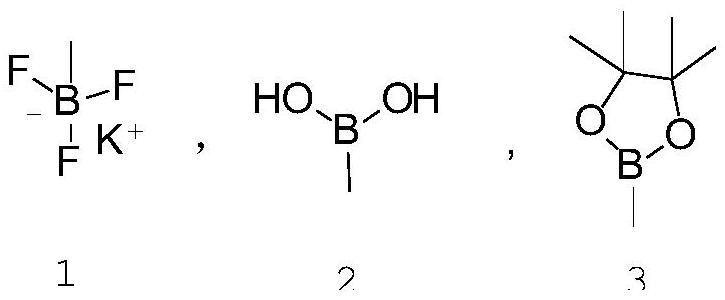
Patents
Literature
35 results about "P-bromophenol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
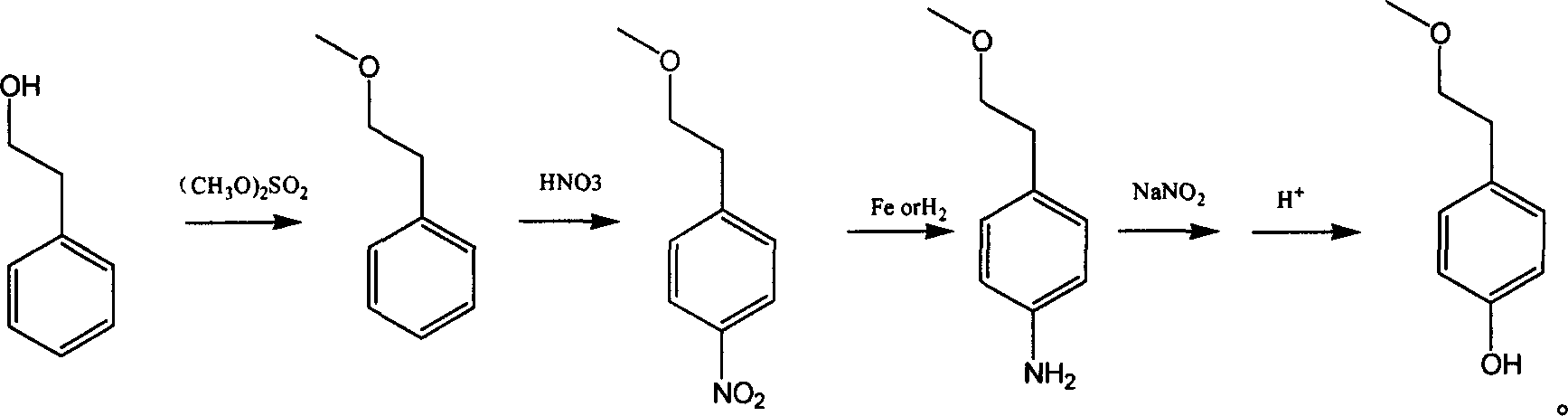
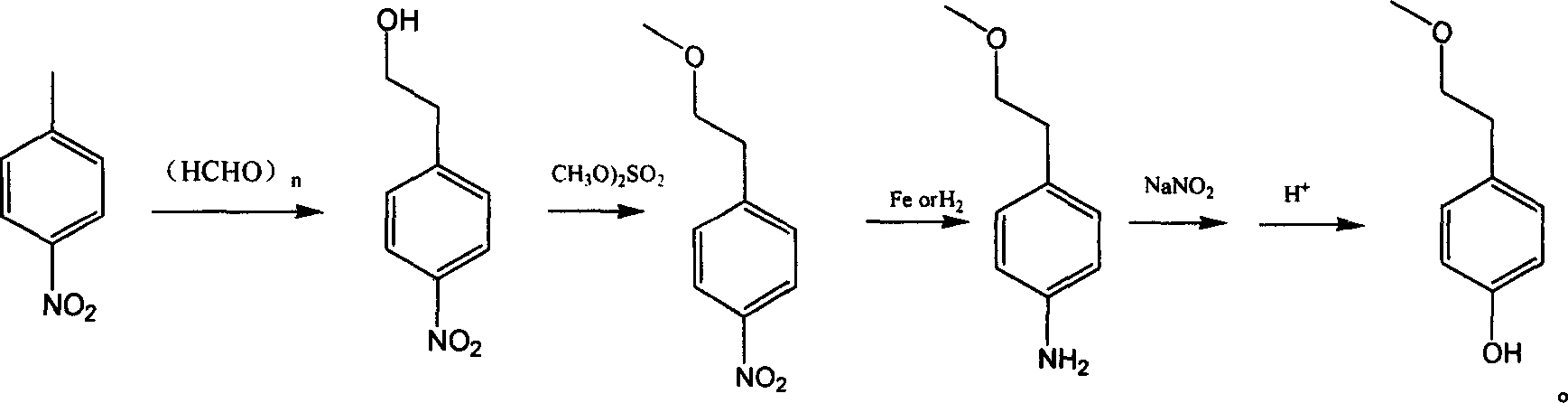
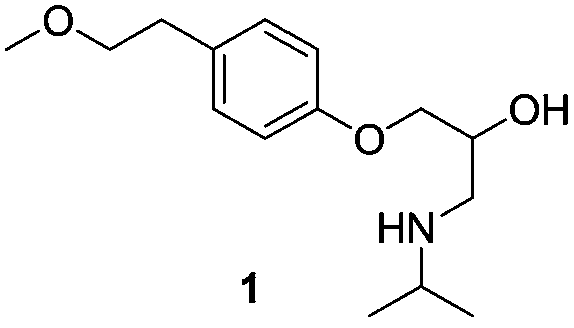
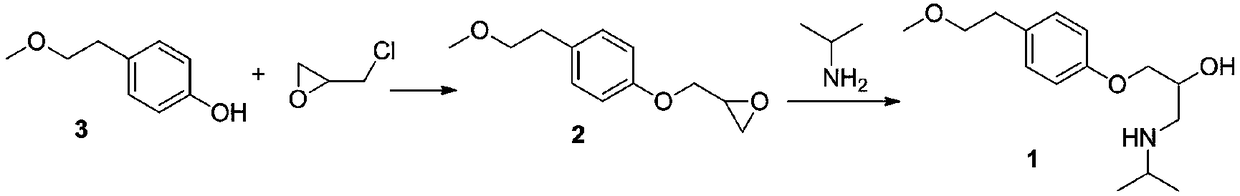
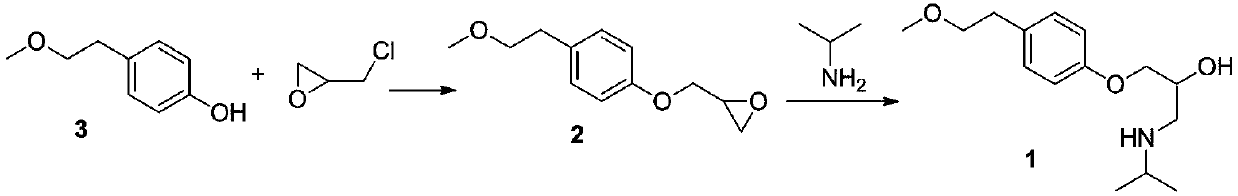
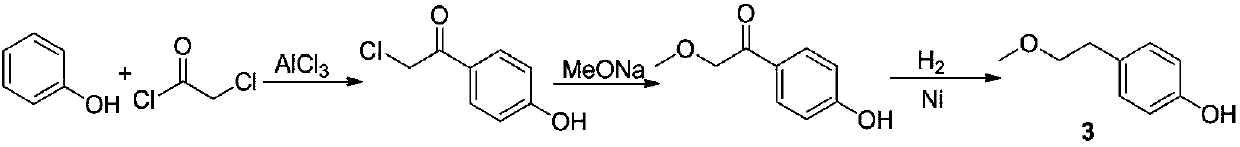
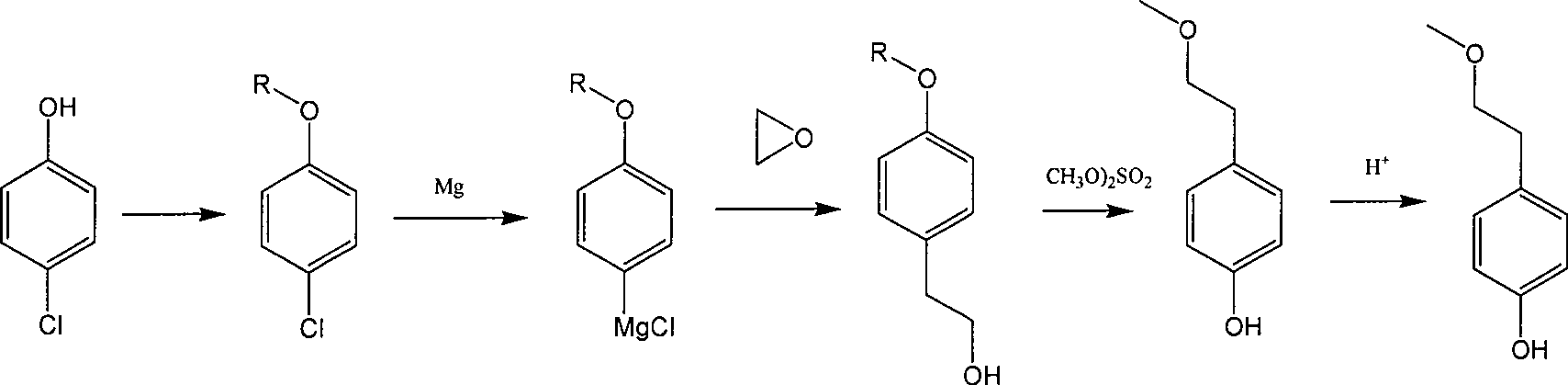
Para-(2-methoxyl) ethylphenol synthesis method
ActiveCN1800128AThe reaction route is simpleThree wastes lessEther preparation from oxiranesTyrosolMethyl carbonate
The invention discloses a method for synthesizing phenetyl, which uses p-chlorophehol or p-bromophenol as starting reaction raw material. It first uses methyl, benzyl or tert-butyl to protect the phenolic hydroxyl, the parivis which is protected by the phenolic hydroxyl reacts with the magnesium in the ether, tetrahydrofuran, tert-butyl methyl ether, isopropyl ether and its mixing solution to obtain the Grignard reagent, the Grignard reagent directly reacted with the etox to obtain the tyrosol which is protected by the phenolic hydroxyl, the tyrosol reacts with the dimethyl sulfate, dimethyl carbonate, trimethyl orthoformate to obtain the tyrosol ether which is protected by phenolic hydroxyl, which obtains the product in acid or hydrogenation protection.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
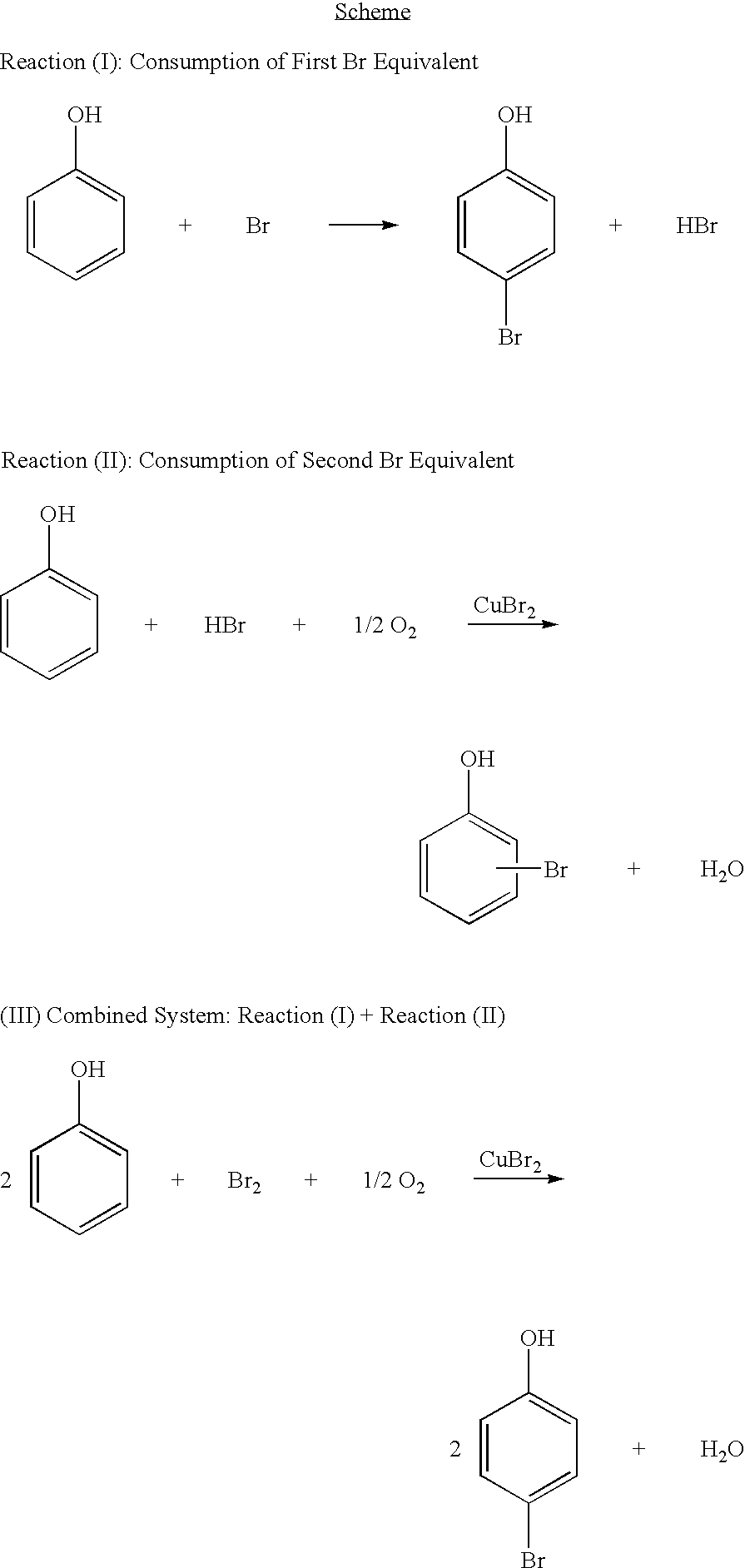
Bromination of hydroxyaromatic compounds and further conversion to dihydroxyaromatic compounds
InactiveCN1756729AOrganic chemistryOrganic compound preparationAlkaline earth metalPhysical chemistry
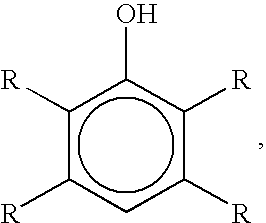
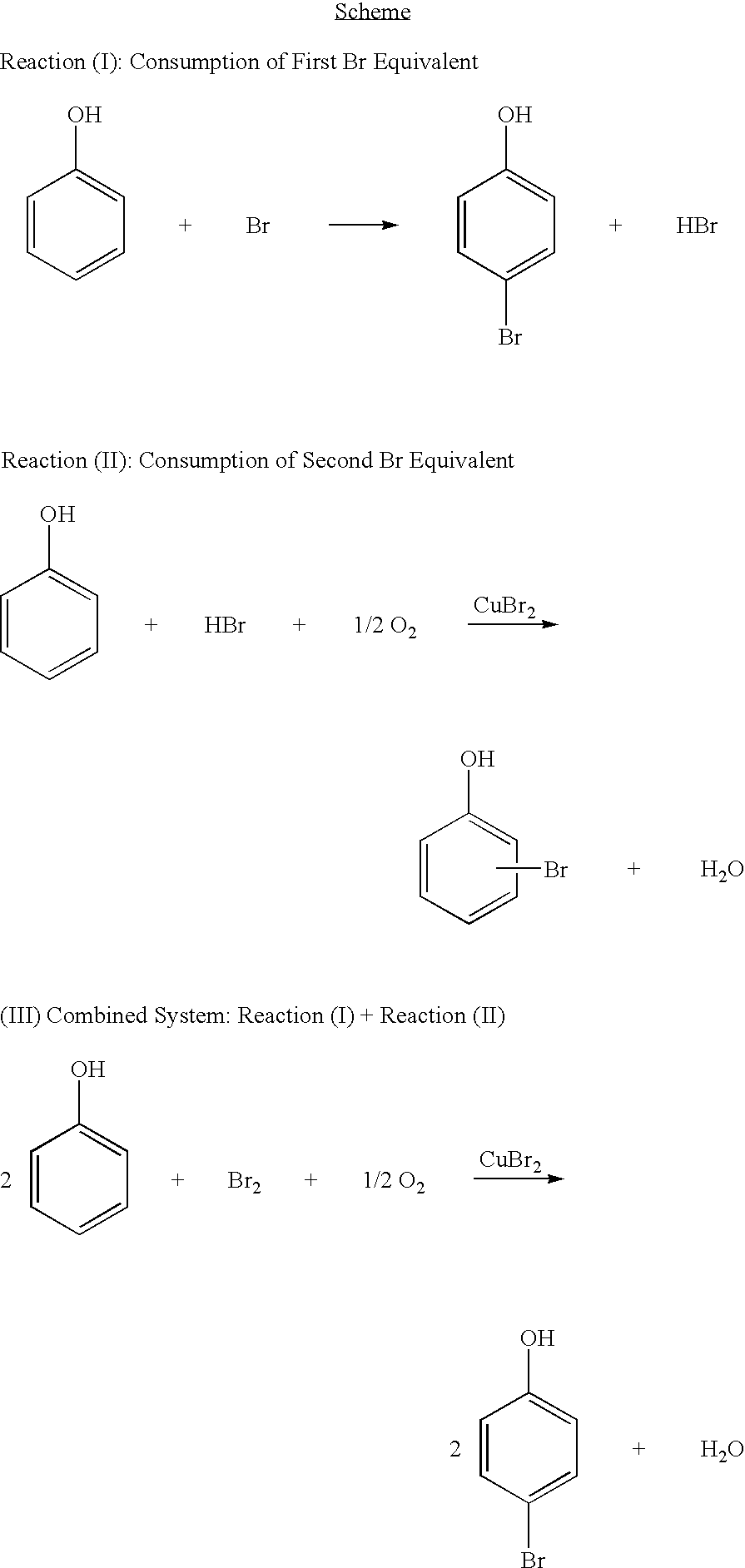
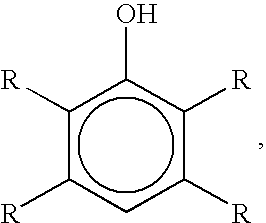
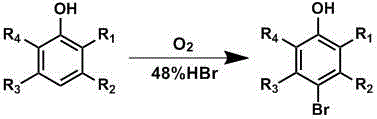
Brominated hydroxyaromatic compounds such as p-bromophenol are prepared by contacting a hydroxyaromatic compound with oxygen and a bromine source such as hydrogen bromide or an alkali metal or alkaline earth metal bromide in an acidic medium, in the presence of elemental copper or a copper compound as catalyst. The brominated product of this reaction may be converted alternately to a dihydroxyaromatic compound such as hydroquinone by hydrolyses, or a dihydroxybiphenyl compound such as 4,4'-dihydroxybiphenyl by reductive coupling.
Owner:SABIC INNOVATIVE PLASTICS IP BV
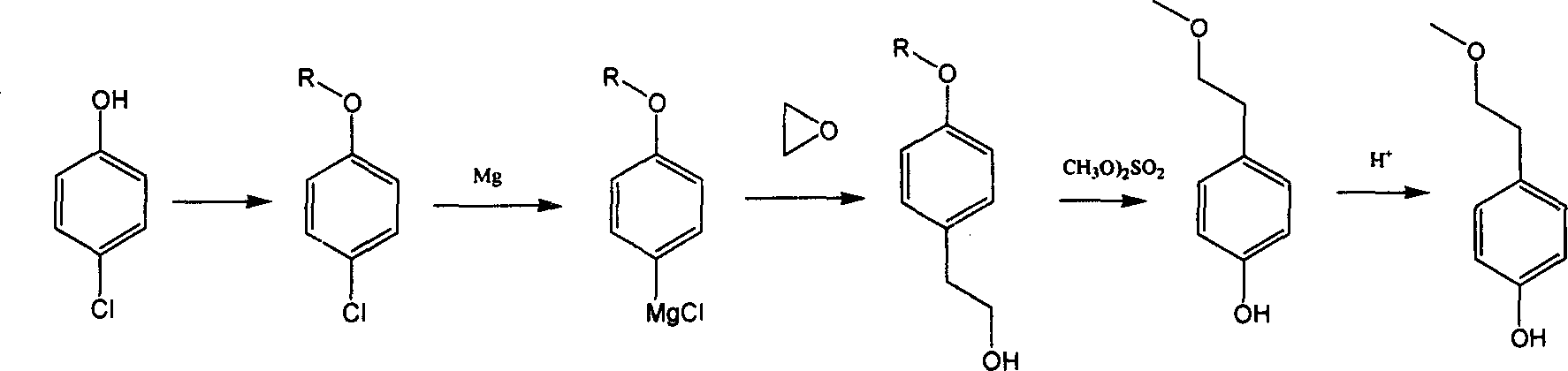
Preparation method of 4-hydroxyphenethyl alcohol
InactiveCN103804147AOrganic compound preparationEther preparation by ester reactionsAlcoholGrignard reaction
The invention relates to a preparation method of 4-hydroxyphenethyl alcohol. The preparation method is characterized in that: p-bromophenol is taken as a raw material, and is subjected to a series of reactions such as hydroxyl protection, Grignard reaction, and protecting group removing so as to obtain 4-hydroxyphenethyl alcohol. Product yield of the preparation method reaches about 90%.
Owner:徐州瑞赛科技实业有限公司
Preparation method of metoprolol intermittent
InactiveCN109369353AHigh yieldHigh purityOrganic chemistryOrganic compound preparationPalladium on carbonOrganic synthesis
The invention belongs to the field of organic synthesis of medicines, and particularly relates to a preparation method of an intermittent of a medicine metoprolol for treating hypertension. A synthesis route of the method comprises the steps that in the presence of a palladium catalyst and a phosphine ligand, p-bromophenol is reacted with methyl vinyl ether to generate 4-(2-methoxy vinyl)phenol; the 4-(2-methoxy vinyl)phenol is subjected to hydrogenation in the presence of a palladium carbon catalyst to obtain the target product 4-(2-methoxyethyl)phenol. The reaction steps are short, the raw materials are cheap and easy to obtain, a technology is simple, operation is convenient, and no special reaction conditions are needed, so that the method is more suitable for industrial production.
Owner:HARVEST PHARMA HUNAN CO LTD
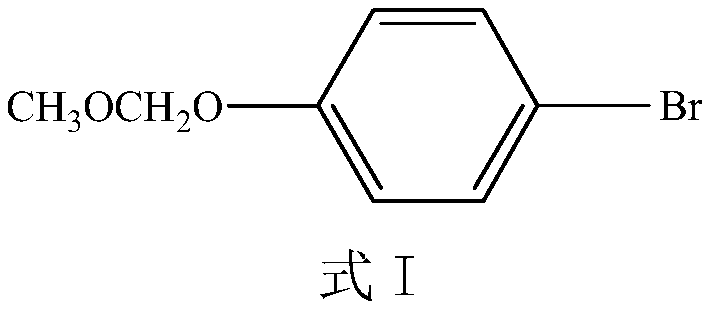
Preparation method of eltrombopag intermediate and preparation method of eltrombopag diethanolamine salt
ActiveCN112979481AHigh yieldHigh synthetic yieldOrganic compound preparationAmino-carboxyl compound preparationPhenylboronic acidNitration
The invention provides a preparation method of an eltrombopag intermediate and a preparation method of eltrombopag diethanolamine salt, and relates to the technical field of medicinal chemistry. According to the preparation method of the eltrombopag intermediate, p-bromophenol is taken as a raw material, and the problem of poor selectivity of nitration reaction is solved. After iodination, the coupling reaction yield of the intermediate compound as shown in a formula (I) and phenylboronic acid is high. Subsequently reduction reaction is carried out, the debromination reaction is complete, the yield is 90% or more, the refining is simpler, and the synthesis yield of the compound of the formula (I) is higher and the operation is simple. Through the improvement, the yield of the key compound shown as the formula (I) is relatively high, the subsequent treatment operation is simple, the process is easy for amplified production, the yield of the whole synthesis process of the eltrombopag diethanolamine salt is 48.1%, the operation is simple, less three wastes are generated, and the synthesis process is suitable for amplified production.
Owner:ZHEJIANG FORESTRY UNIVERSITY
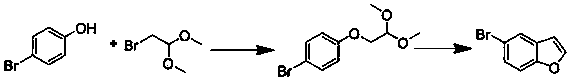
Preparation method of 5-bromobenzofuran
The invention belongs to the field of organic synthesis, and particularly relates to a preparation method of 5-bromobenzofuran. The preparation method of 5-bromobenzofuran comprises the following steps: p-bromophenol and 2-bromoacetaldehyde dimethyl acetal or 2-chloroacetaldehyde dimethyl acetal react for several hours at a proper temperature in the presence of proper solvent and alkali to generate 1-bromo-4'-(2,2-dimethoxyethyl) benzene; the 1-bromo-4'-(2,2-dimethoxyethyl) benzene is heated in the corresponding solvent in the presence of acid, and experiences a cyclization reaction; the product is purified to obtain 5-bromobenzofuran. The method provided by the invention has the beneficial effects that 5-bromobenzofuran is prepared by a two-step process in the reaction, the operation is simple and convenient, the production cost is reduced, and industrial large-scale production is easy to realize; meanwhile, environmental pollution caused by a heavy metal catalyst is avoided.
Owner:SHANDONG YOUBANG BIOCHEM TECH
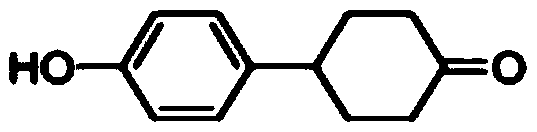
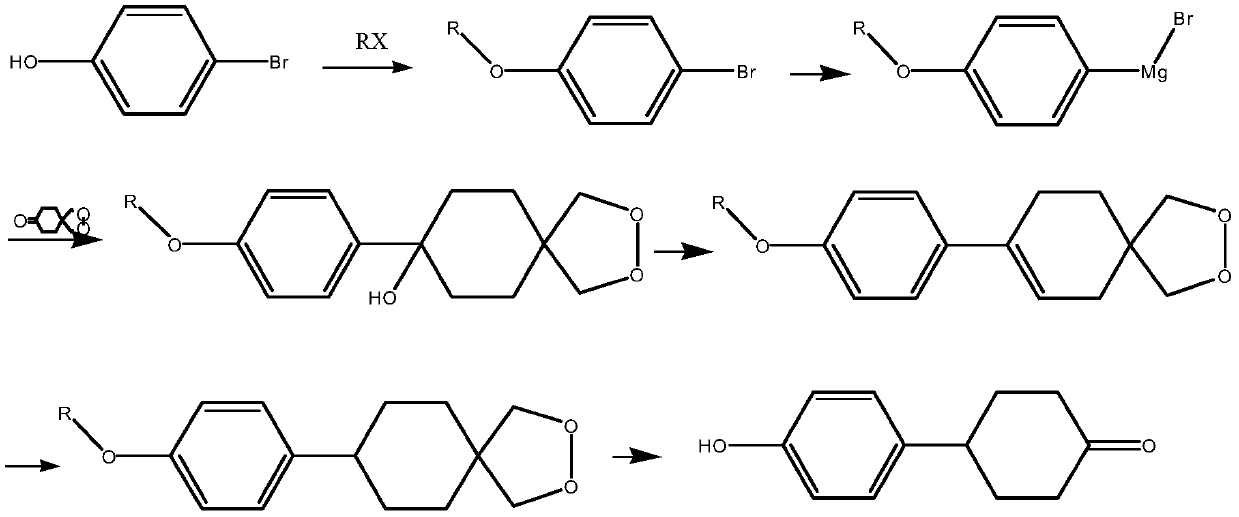
Preparation method of 4-(4-hydroxyphenyl) cyclohexanone
InactiveCN110028395ARealize industrial productionReasonable process designGroup 4/14 element organic compoundsOrganic compound preparationCyclohexanoneChemical synthesis
The invention relates to the technical field of chemical synthesis, in particular to a preparation method of 4-(4-hydroxyphenyl) cyclohexanone. According to the preparation method, p-bromophenol is used as a raw material, a Grignard reagent is prepared through hydroxyl protection and Grignard reaction, then the prepared Grignard reagent is utilized to couple with 1,4-cyclohexanedione monoethyleneglycol ketal, then the product is dehydrated to obtain alkene, and double protecting groups are removed after hydrogenation to prepare a target product, namely, 4-(4-hydroxyphenyl) cyclohexanone. Thepreparation method of 4-(4-hydroxyphenyl) cyclohexanone, provided by the invention, has the advantages of reasonable process design, high yield and low production cost; the prepared 4-(4-hydroxyphenyl) cyclohexanone is a white solid, the HPLC content is more than 99.5%, the total yield of the product can reach 75-80%, and the raw materials are easy to obtain; and the method has simple operation and high safety, and realizes industrial production of 4-(4-hydroxyphenyl) cyclohexanone.
Owner:宁夏中星显示材料有限公司
Bromination of hydroxyaromatic compounds
ActiveUS20050049440A1Eliminates formationSpeed up the conversion processOrganic compound preparationOrganic halogenationCompound aMetal catalyst
A method for brominating hydroxyaromatic compounds to form products, such as p-bromophenol, is disclosed. The method uses elemental bromine as the brominating agent and comprises contacting a hydroxyaromatic compound with bromine and oxygen in the presence of metal catalyst. Suitable catalysts include elemental copper, copper compounds, and compounds of Group IV-VIII transition metals.
Owner:SHPP GLOBAL TECH BV
Preparation method of intermediate of metoprolol
ActiveCN109553513AShort process routeProcess reaction conditions are mildOrganic chemistryOrganic compound preparationPalladium on carbonOrganic synthesis
The invention belongs to the field of organic synthesis of medicines, and particularly relates to a preparation method of an intermediate of a medicine metoprolol for treating hypertension. The synthetic route provided by the invention is as follows: p-bromophenol reacts with methyl vinyl ether in the presence of a palladium catalyst and a phosphine ligand to generate 4-(2-methoxyvinyl)phenol; andhydrogenating the 4-(2-methoxy vinyl)phenol in the presence of a palladium-carbon catalyst to obtain the target product 4-(2-methoxyethyl)phenol. The reaction steps of the method are short, the raw materials are cheap and are easily available, the process is simple, operation is convenient, no special reaction conditions are needed, and therefore, the method is more suitable for industrial production.
Owner:HARVEST PHARMA HUNAN CO LTD
Preparation method of 4-bromophenetole
InactiveCN101913999AAvoid investmentEliminate cryogenic freezing stepEther separation/purificationEther preparation by ester reactionsWater vaporPotassium hydroxide
The invention relates to a preparation method of 4-bromophenetole. The method comprises (1) an etherification reaction step: putting p-bromophenol and diethyl sulfate into a reaction kettle, stirring, heating and continuously adding potassium hydroxide to react; (2) a washing step: adding water to the mixture after the etherification reaction, evenly stirring, standing still to delaminate and separate oily liquid; and (3) a distillation step: introducing vapor in the oily layer for vapor distillation to obtain the finished product of 4-bromophenetole. By using a vapor distillation technology and potassium hydroxide, the preparation method reduces the feeding amounts of diethyl sulfate and water, saves the cost of the raw materials, improves the product quality and enhances the 4-bromophenetole content to 99.5 percent.
Owner:TIANJIN CHEM REAGENT RES INST
Preparation method of esmolol hydrochloride intermediate
PendingCN111620782AHigh yieldHigh purityOrganic compound preparationCarboxylic acid esters preparationPalladium on carbonPtru catalyst
The invention belongs to the field of organic synthesis of medicines, and particularly relates to a preparation method of a medicine esmolol hydrochloride intermediate for treating hypertension. The synthesis route provided by the invention comprises the following steps: reacting p-bromophenol with methyl acrylate in the presence of a palladium catalyst and a phosphine ligand to generate 3-(4-hydroxyphenyl) methyl acrylate; and hydrogenating the generated 3-(4-hydroxyphenyl) methyl acrylate in the presence of a palladium-carbon catalyst to obtain a target product methyl 4-hydroxyphenylpropionate. The method has the advantages of short reaction steps, cheap and accessible raw materials, simple technique and convenience of operation, and does not need special reaction conditions, thereby being more suitable for industrial production.
Owner:HARVEST PHARMA HUNAN CO LTD
Method for preparation of para-brominated hydroxyaromatic compounds
InactiveUS20050059843A1Eliminate needImprove responseOrganic chemistryMolecular sieve catalystsPtru catalystMetal catalyst
A method for preparing hydroxyaromatic compounds brominated in the para-position, such as p-bromophenol, is disclosed. The method yields overall high process selectivity through isomeric equilibration and separation of the brominated products, thereby eliminating the need for high para selectivity in the products of catalytic oxybromination reactions of hydroxyaromatic compounds using oxygen, a bromine source, and an acidic medium in the presence of a metal catalyst. Furthermore, the invention provides an efficient method for recycling the metal catalyst, as well as reagents used in the bromination, to further reactions.
Owner:SABIC INNOVATIVE PLASTICS IP BV
Synthetic method of p-bromophenol
InactiveCN102863317AHigh yieldSimple processOrganic chemistryOrganic compound preparationMagnetiteElectromagnetic heating
The invention relates to a synthetic method of p-bromophenol, which belongs to the technical field of a chemical reagent synthetic method, and especially relates to the synthetic method of p-bromophenol. The invention provides the synthetic method of p-bromophenol with high yield of p-bromophenol, simple technology and easy industrialization. The method comprises the following steps: 1) weighting a certain amount of phenol, dissolving in a quantitative solvent, adding in a 4-mouth flask, then putting the 4-mouth flask in a reaction tank; 2) adding a magnetite in the 4-mouth flask, turning on an electromagnetic heating stirrer for stirring, inserting a thermometer and a liquid dropping tunnel in the 4-mouth flask; 3) weighting the certain amount of bromine and the quantitative solvent and dumping into the liquid dropping tunnel, adding ice cubes or iced brine in the reaction tank, controlling the temperature, when the temperature constantly reaches the predetermined temperature of an experiment, opening the liquid dropping tunnel, adding the bromine solution drop by drop, and then timing right now.
Owner:俞龙飞
Method for preparation of para-brominated hydroxyaromatic compounds
InactiveUS6982356B2Improve responseHigh selectivityOrganic chemistryMolecular sieve catalystsPtru catalystMetal catalyst
Owner:SABIC INNOVATIVE PLASTICS IP BV
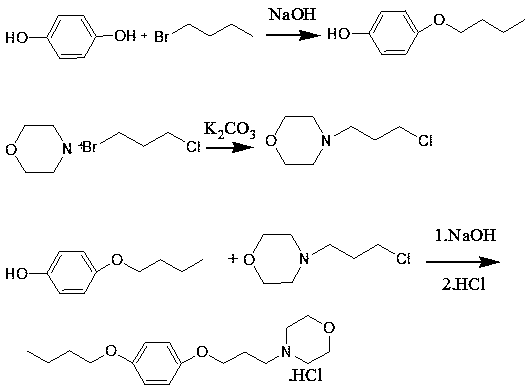
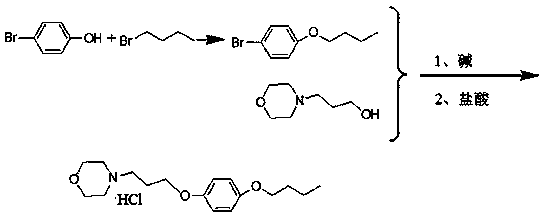
A pramoxine hydrochloride preparing method
The invention belongs to the technical field of medicine synthesis, and particularly relates to a pramoxine hydrochloride preparing method. The method includes reacting p-bromophenol and 1-bromobutaneunder catalytic functions of an alkali to prepare an intermediate that is 1-bromo-4-tert-butoxybenzene; synthesizing pramoxine having a base group from the 1-bromo-4-tert-butoxybenzene and 3-morpholinopropanol with the existence of a strong alkali; and subjecting the pramoxine having the base group to salification to obtain the pramoxine hydrochloride. The yield of the pramoxine hydrochloride prepared by the method is higher than 80%, and liquid-phase purity of the prepared pramoxine hydrochloride is higher than 99.8%. The method is used for preparing the pramoxine hydrochloride, and is simple to operate and high in yield, raw materials are easily available, product purity is high, the cost is low, and pollution is low.
Owner:山东诚汇双达药业有限公司
Bromination of hydroxyaromatic compounds
ActiveUS7053251B2Eliminates formationSpeed up the conversion processOrganic compound preparationOrganic halogenationCompound aMetal catalyst
A method for brominating hydroxyaromatic compounds to form products, such as p-bromophenol, is disclosed. The method uses elemental bromine as the brominating agent and comprises contacting a hydroxyaromatic compound with bromine and oxygen in the presence of metal catalyst. Suitable catalysts include elemental copper, copper compounds, and compounds of Group IV–VIII transition metals.
Owner:SHPP GLOBAL TECH BV
Preparation method of p-bromoethoxybenzene
InactiveCN101891600AAvoid investmentEliminate cryogenic freezing stepEther preparation by ester reactionsDistillationReaction temperature
The invention relates to a preparation method of p-bromoethoxybenzene, comprising the following steps: (1) etherification reaction: throwing p-bromophenol and diethyl sulfate into a reaction kettle, stirring, heating, distributing and adding sodium hydroxide to react; (2) washing: adding water to a mixture after etherification reaction, evenly stirring, standing and layering to separate out oily liquid; and (3) rectification: rectifying an oil layer by an efficient rectification tower to obtain the finished product. In the preparation method, the efficient rectification tower is used for rectification instead of the original distillation operation, thus reducing the usage amount of diethyl sulfate and water, keeping the reaction temperature at 30-50 DEG C, saving energy, obtaining high-quality and high-content p-bromoethoxybenzene, achieving high content of p-bromoethoxybenzene up to 99.5% and enhancing the market competitiveness of the product.
Owner:TIANJIN CHEM REAGENT RES INST
Method for preparing 4-hydroxy-4'-cyanobiphenyl
ActiveCN109912455ASmall market capacityHigh priceCarboxylic acid nitrile preparationOrganic compound preparationOrganic synthesisCombinatorial chemistry
The invention relates to a method for preparing 4-hydroxy-4'-cyanobiphenyl, and belongs to the technical field of organic synthetic chemistry. According to the method, substituted boronic acid is first prepared by taking p-bromophenol as a raw material, and then the substituted boronic acid is prepared into the 4-hydroxy-4'-cyanobiphenyl, wherein PG in the substituted boronic acid is a protectinggroup, the substituted boronic acid and p-chlorobenzonitrile are subjected to a Suzuki coupling reaction in the presence of a solvent, an alkali and a catalyst, deprotection is performed to obtain the4-hydroxy-4'-cyanobiphenyl, the molar ratio of p-bromophenol, p-chlorobenzonitrile and the alkali is 1.0:(1.0-1.5):(2.0-4.0), and the temperature of the Suzuki coupling reaction is 50-120 DEG C. Themethod provided by the invention has the advantages that the production efficiency is high, three wastes are fewer, raw materials are easy to obtain, the cost is low, and the obtained product, namely,4-hydroxy-4'-cyanobiphenyl, is high in yield.
Owner:宁夏中星显示材料有限公司
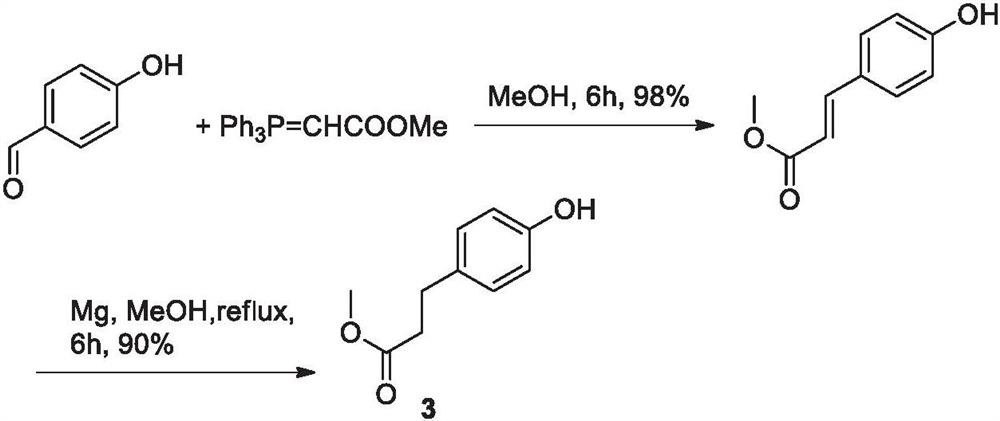
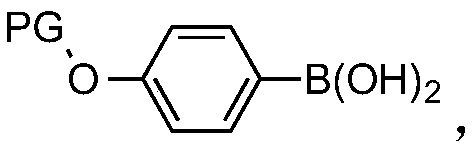
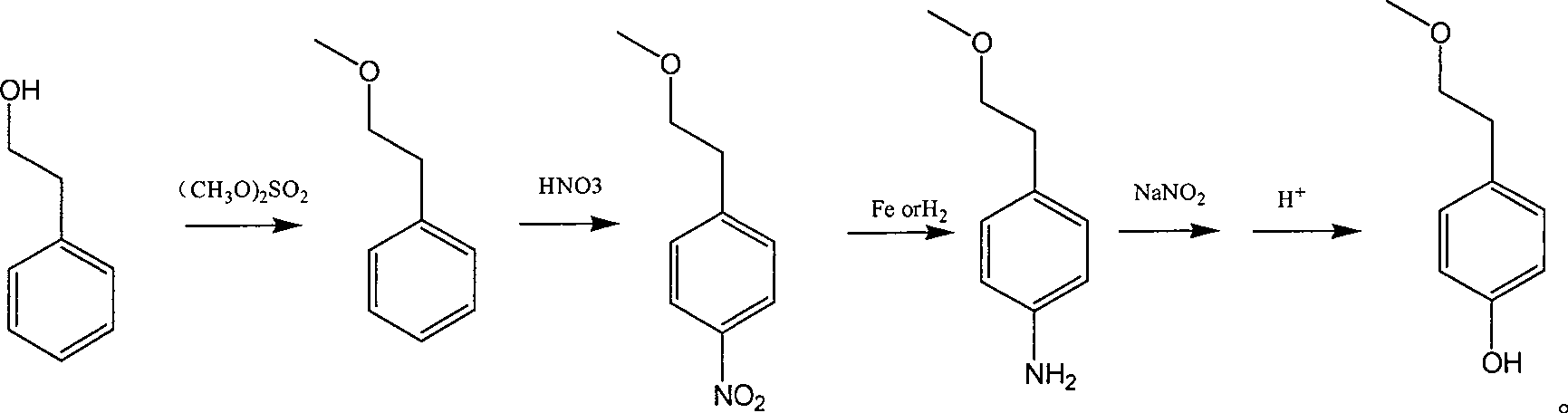
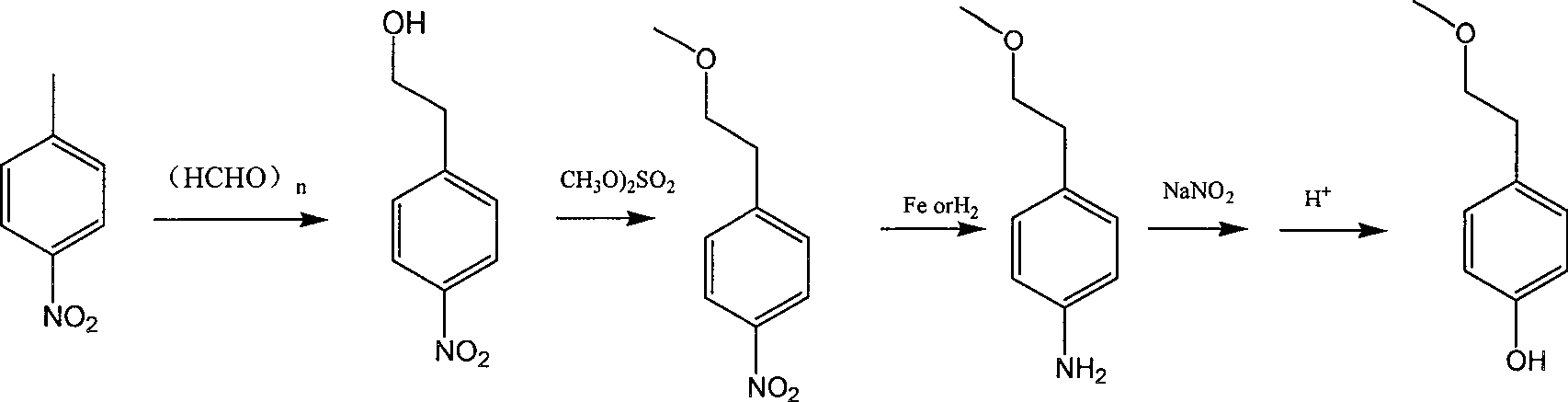
Para-(2-methoxyl) ethylphenol synthesis method
ActiveCN100482629CThe reaction route is simpleThree wastes lessEther preparation from oxiranesTyrosolMethyl carbonate
The invention discloses a method for synthesizing phenetyl, which uses p-chlorophehol or p-bromophenol as starting reaction raw material. It first uses methyl, benzyl or tert-butyl to protect the phenolic hydroxyl, the parivis which is protected by the phenolic hydroxyl reacts with the magnesium in the ether, tetrahydrofuran, tert-butyl methyl ether, isopropyl ether and its mixing solution to obtain the Grignard reagent, the Grignard reagent directly reacted with the etox to obtain the tyrosol which is protected by the phenolic hydroxyl, the tyrosol reacts with the dimethyl sulfate, dimethyl carbonate, trimethyl orthoformate to obtain the tyrosol ether which is protected by phenolic hydroxyl, which obtains the product in acid or hydrogenation protection.
Owner:SHANDONG HANXING PHARM TECH CO LTD +1
Bromination of hydroxyaromatic compounds and further conversion to dihydroxyaromatic compounds
InactiveUS7045666B2Organic chemistryOrganic compound preparationAlkaline earth metalHydroquinone Compound
Brominated hydroxyaromatic compounds such as p-bromophenol are prepared by contacting a hydroxyaromatic compound with oxygen and a bromine source such as hydrogen bromide or an alkali metal or alkaline earth metal bromide in an acidic medium, in the presence of elemental copper or a copper compound as catalyst. The brominated product of this reaction may be converted alternately to a dihydroxyaromatic compound such as hydroquinone by hydrolyses, or a dihydroxybiphenyl compound such as 4,4′-dihydroxybiphenyl by reductive coupling.
Owner:SHPP GLOBAL TECH BV
Preparation method of p-bromophenol compound
The present invention provides a preparation method of p-bromophenol compound. The method is characterized in that high selectivity preparation of p-bromophenol is conducted on the raw material phenol compound by using a transition metal catalyst in an oxygen-containing atmosphere.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
A kind of synthetic method of 2,5-dibromoiodobenzene
ActiveCN109096043BLow costExistence is largeOrganic compound preparationAmino compound preparationBenzeneChemical reaction
Owner:鹤壁市海格化工科技有限公司
Synthetic method of p-acetoxystyrene
PendingCN112409176AReduce pollutionMild reaction conditionsOrganic compound preparationCarboxylic acid esters preparationBiochemical engineeringBoronic acid
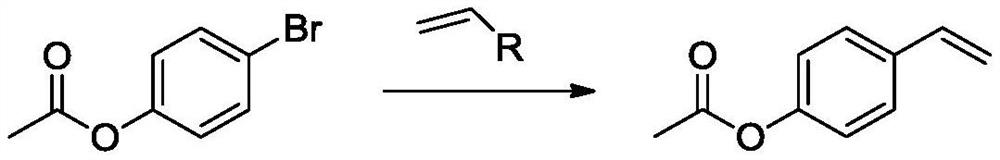
The invention discloses a synthesis method of p-acetoxystyrene, which comprises the following steps: carrying out acetylation reaction on p-bromophenol to generate 4-acetoxybromobenzene, and reactingthe 4-acetoxybromobenzene with a vinyl boric acid compound to obtain p-acetoxystyrene. The method is mild in reaction condition, high in yield, environmentally friendly and suitable for industrial production.
Owner:惠泽化学科技(濮阳)有限公司
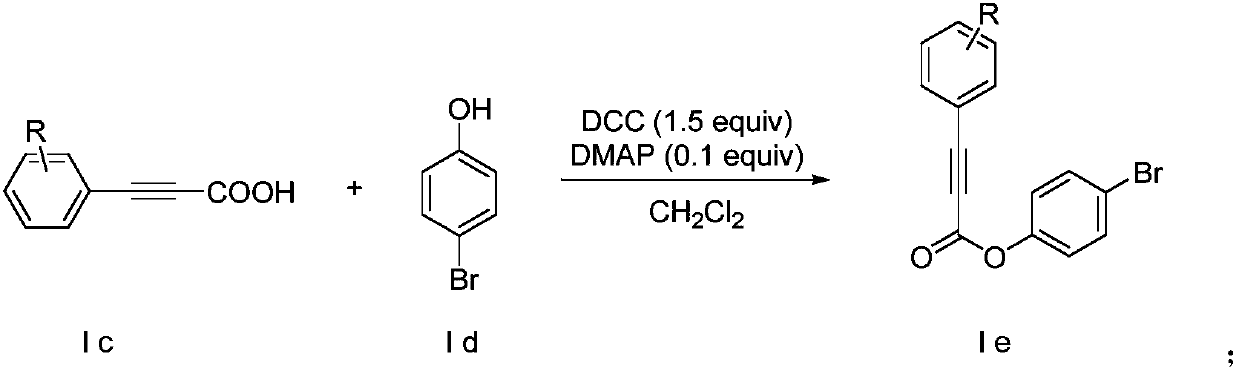
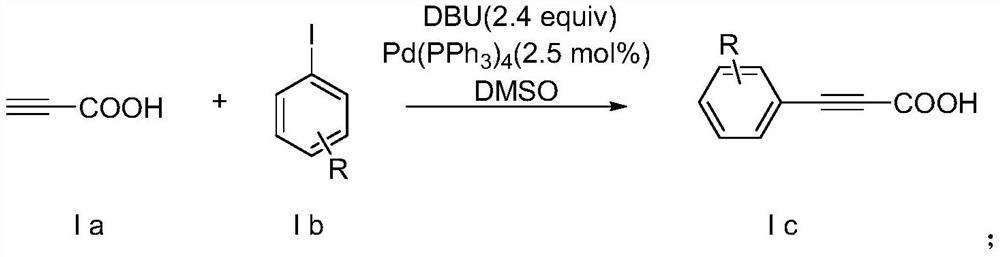
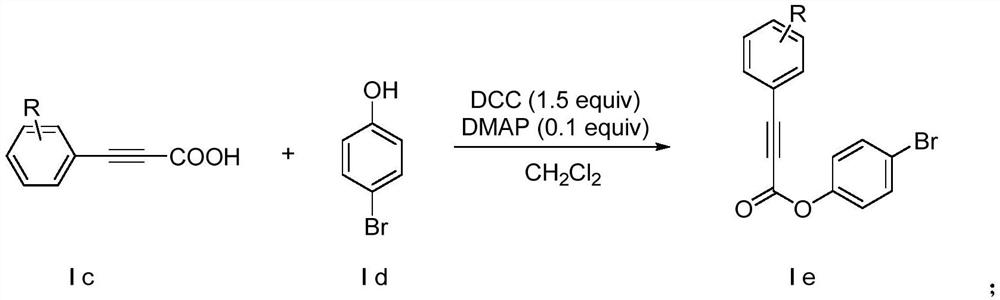
Method for synthesizing alpha-alkynyl substituted ether compounds
ActiveCN107556269AImprove compatibilityThe synthesis method is simple and easy to obtainOrganic chemistryPhenolPropiolic acid
The invention discloses a method for preparing alpha-alkynyl substituted ether compounds. The method comprises the following steps: taking propiolic acid as a raw material, and synthesizing substituted phenylpropiolic acid under conditions of substituted iodobenzene, 1,8-diazabicyclo[5.4.0]undec-7-ene, (beta-4)-platinu and dimethyl sulfoxide; taking substituted phenylpropiolic acid and p-bromophenol as raw materials, and synthesizing p-bromophenyl substituted phenylpropiolic acid ester under the conditions of 4-dimethylaminopyridine and N,N'-Dicyclohexylcarbodiimide; and performing an alkynylation reaction on the p-bromophenyl substituted phenylpropiolic acid ester in participation of tert-butyl hydroperoxide, cesium carbonate and tetrahydrofuran, and finally synthesizing alpha-alkynyl substituted ether products. According to the method disclosed by the invention, simple and readily available acetylene esters serve as an alkynylation reagent, and the target products, namely alpha-alkynyl substituted ether compounds, are synthesized in a green and environmental-friendly manner under mild conditions. The compounds play an important role in construction of multiple medical intermediates and bio-active structures.
Owner:NANJING UNIV OF SCI & TECH
Synthetic method of α-alkynyl substituted ether compounds
ActiveCN107556269BImprove compatibilityThe synthesis method is simple and easy to obtainOrganic chemistryCarbenePropiolic acid
The invention discloses a method for preparing alpha-alkynyl substituted ether compounds. The method comprises the following steps: taking propiolic acid as a raw material, and synthesizing substituted phenylpropiolic acid under conditions of substituted iodobenzene, 1,8-diazabicyclo[5.4.0]undec-7-ene, (beta-4)-platinu and dimethyl sulfoxide; taking substituted phenylpropiolic acid and p-bromophenol as raw materials, and synthesizing p-bromophenyl substituted phenylpropiolic acid ester under the conditions of 4-dimethylaminopyridine and N,N'-Dicyclohexylcarbodiimide; and performing an alkynylation reaction on the p-bromophenyl substituted phenylpropiolic acid ester in participation of tert-butyl hydroperoxide, cesium carbonate and tetrahydrofuran, and finally synthesizing alpha-alkynyl substituted ether products. According to the method disclosed by the invention, simple and readily available acetylene esters serve as an alkynylation reagent, and the target products, namely alpha-alkynyl substituted ether compounds, are synthesized in a green and environmental-friendly manner under mild conditions. The compounds play an important role in construction of multiple medical intermediates and bio-active structures.
Owner:NANJING UNIV OF SCI & TECH
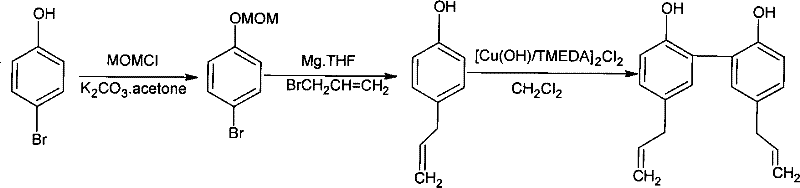
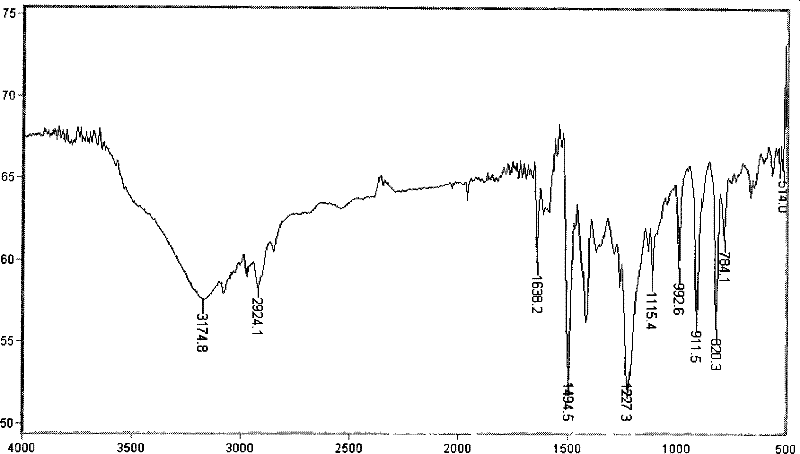
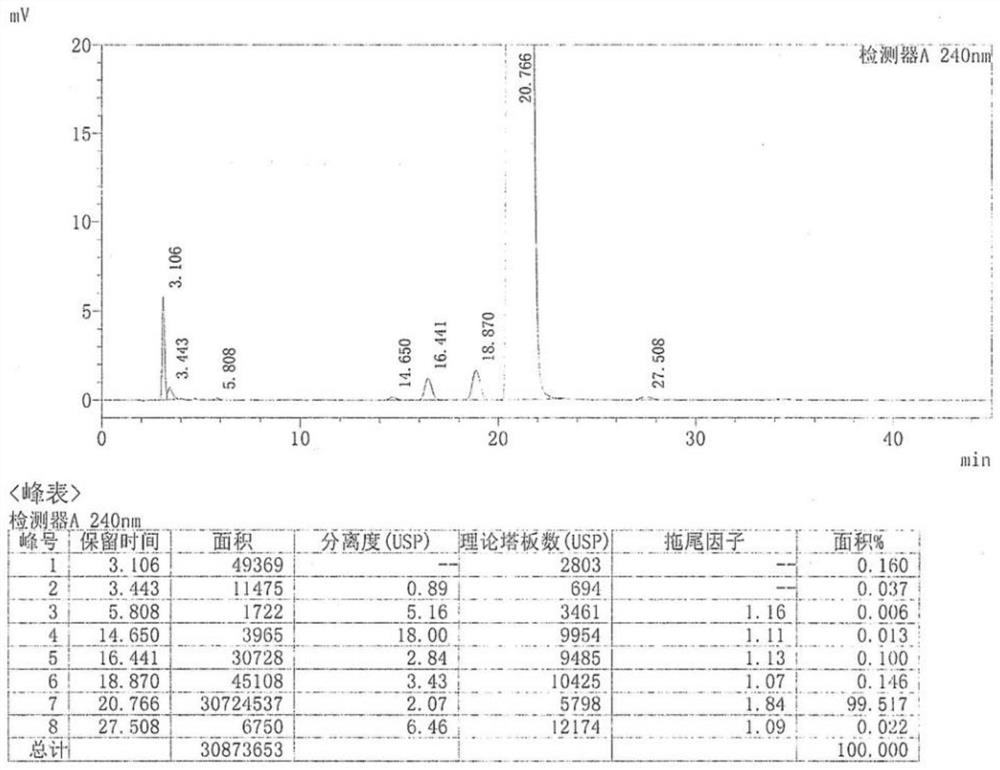
Magnolol synthesizing method
InactiveCN101293816BHigh yieldOxidative coupling reaction is not easy to occurOrganic chemistryOrganic compound preparationChemical synthesisMagnolol
The present invention provides a method for synthesizing magnolol. The magnolol is prepared from relatively cheap p-bromophenol, chloromethyl methyl ether and allyl bromide by being subjected to hydroxy protection and Grignard reaction to obtain allylphenol intermediate product; dissolving allylphenol into solvent; and carrying out oxidation coupling reaction in the presence of oxidation catalyst and introducing air or oxygen. The method is characterized in that the adopted catalyst is hydroxy*tetramethyl ethylene diamine copper (I) dichloride and has a molecular formula of [Cu(OH) / TMEDA]2Cl2, which allows p-allylphenol to carry out oxidation coupling reaction effectively and solves the problem of difficulty in industrial production of magnolol chemical synthesis; and the total yield of the synthesized magnolol product can reach 50%. The method has the advantages of simple synthetic process, high yield and low cost, and can be applied in industrial production. Comparing test results of infrared detection and high performance liquid phase analysis and detection with those of the magnolol product extracted from plants, the synthesized magnolol product has the similar or the same structure.
Owner:JIANGNAN UNIV
Industrial preparation process of tamoxifen citrate
PendingCN114133334AEasy to recycleReduce unit consumptionOrganic compound preparationAmino-hyroxy compound preparationGrignard reagentGrignard reaction
The invention relates to the technical field of medicine synthesis, in particular to an industrial preparation process of tamoxifen citrate. The preparation process comprises the following steps: (1) etherification: taking p-bromophenol and dimethylaminochloroethane hydrochloride as raw materials, taking methylbenzene as a solvent, and carrying out etherification reaction under the action of sodium hydroxide to obtain etherate; (2) Grignard reaction: by taking tetrahydrofuran as a solvent, reacting the etherate with an initiator to obtain a Grignard reagent, and then carrying out Grignard reaction on the Grignard reagent and alpha-ethyl desoxybenzoin to obtain a Grignard substance; (3) dehydration and alkalization: carrying out a dehydration reaction on the Grignard substance and hydrochloric acid, and then carrying out an alkalization reaction with a sodium hydroxide solution to obtain tamoxifen free alkali; and (4) salt forming reaction: taking the tamoxifen free alkali and citric acid as raw materials, taking acetone as a solvent, and carrying out salt forming reaction to obtain the tamoxifen citrate. The process conditions are easy to control, the product purity reaches 99.5%, the total yield of the product is high, and the production cost is low.
Owner:BEIJING JINGFENG PHARM (SHANDONG) CO LTD
A novel sulfonyl chloride derivative, its preparation method and its application
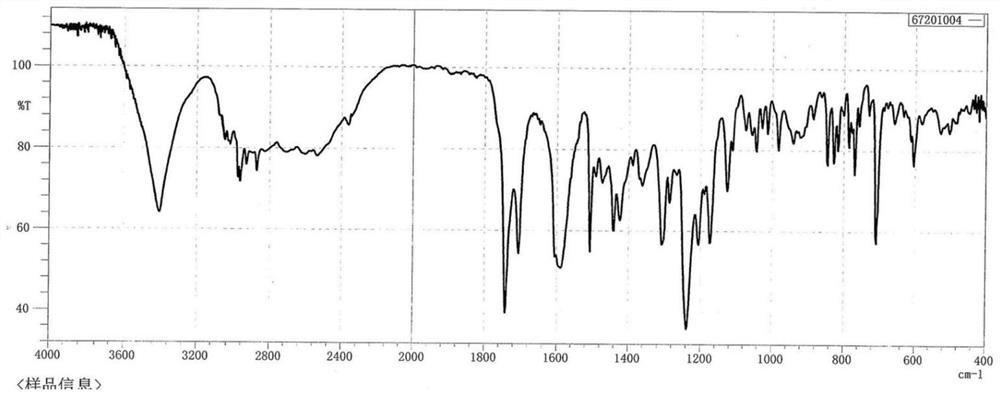
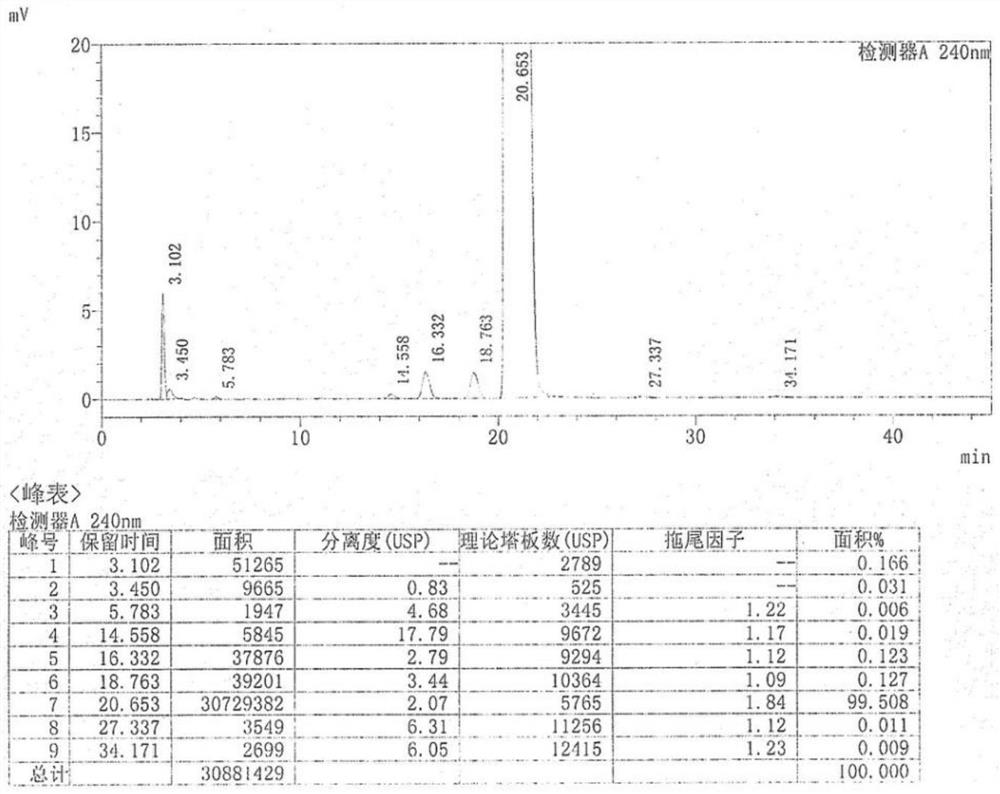
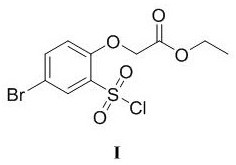
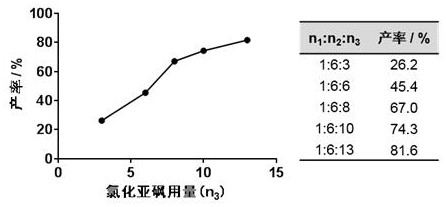
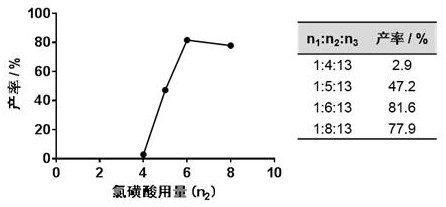
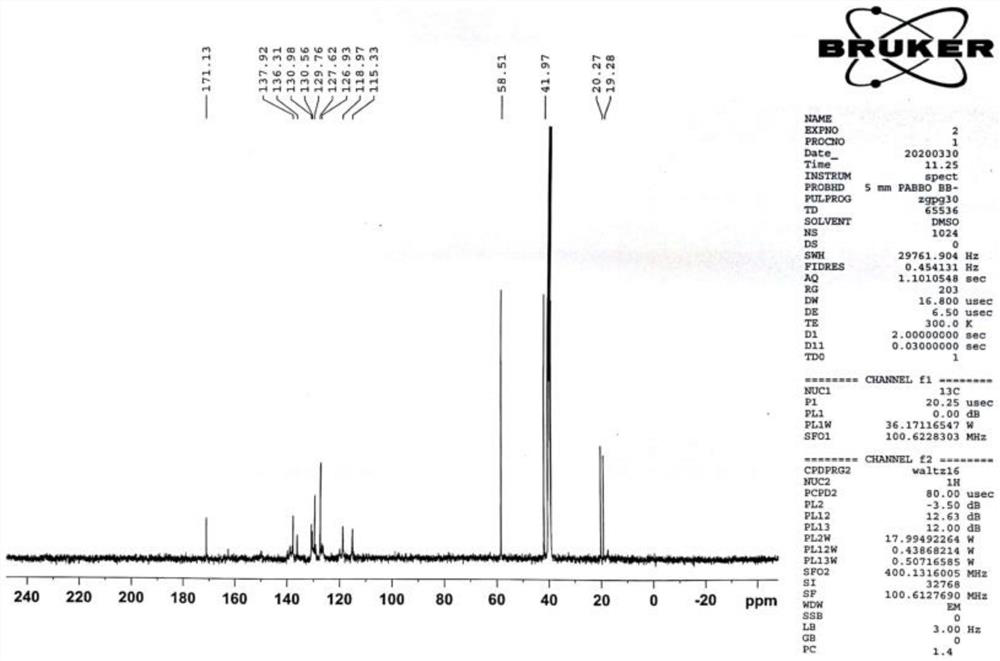
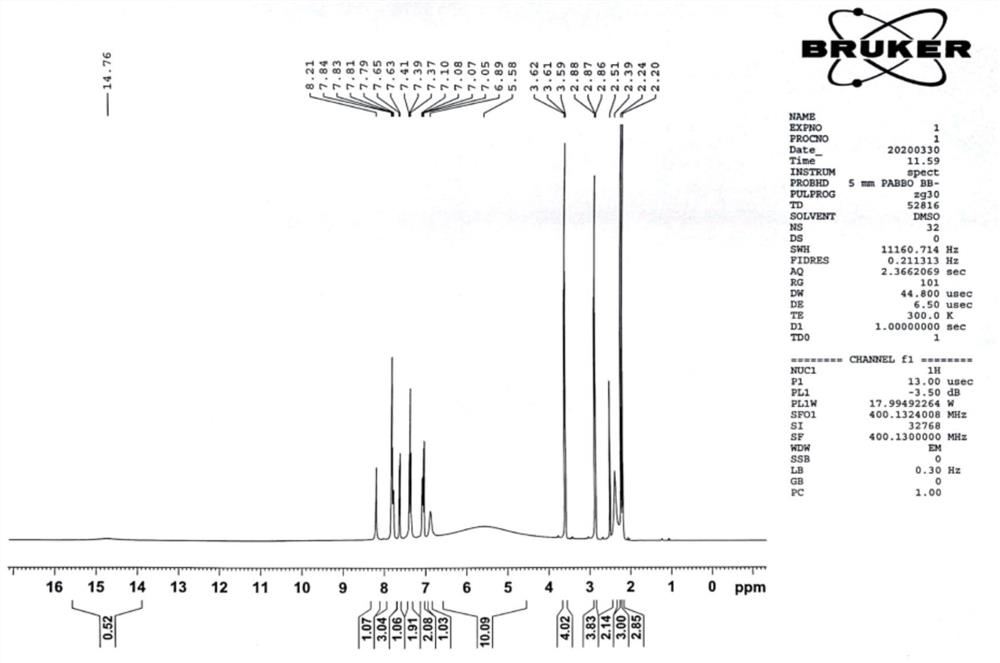
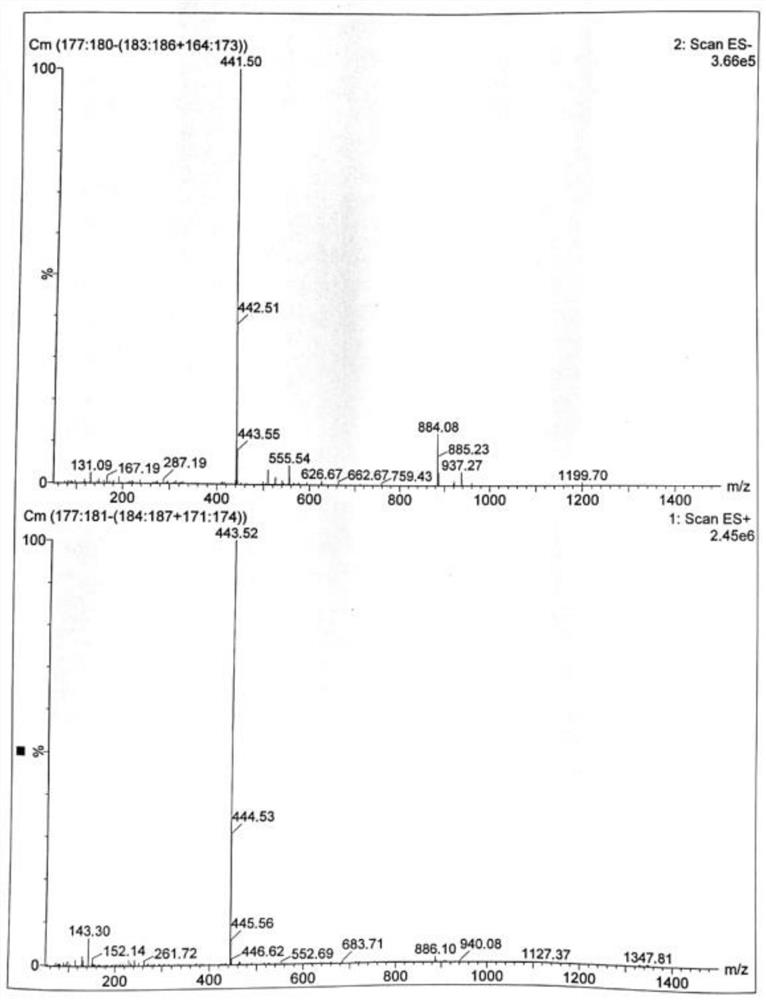
ActiveCN110483343BHigh yieldStarting materials are cheap and readily availableOrganic compound preparationCarboxylic acid esters preparationSulfonyl chlorideChlorosulfuric acid
The present invention provides a novel sulfonyl chloride derivative, its preparation method and application. The structural formula of the sulfonyl chloride derivative is shown in Formula I. The preparation method comprises the following steps: 1) taking compound 1, i.e. p-bromophenol as a raw material, under the catalysis of a base, in a suitable organic solvent, substituting the phenolic hydroxyl group with ethyl chloroacetate to obtain compound 2, That is, 2-(4-bromophenoxy)ethyl acetate; 2) In a suitable organic solvent, the compound 2 is chlorosulfonated on the benzene ring with chlorosulfonic acid and a chlorinating reagent to obtain the compound of formula I. This derivative and its synthesis process have not seen any domestic and foreign literature reports at present, and there is no similar product on the market. The present invention designs a brand-new synthetic route, and optimizes the conditions for the key reaction steps in the route, and determines the optimal process conditions. The new process has the characteristics of cheap and easy-to-obtain starting materials, simple steps, easy post-processing and high yield, and is suitable for industrial production.
Owner:TAIZHOU POLYTECHNIC COLLEGE
A kind of preparation method of Eltrombopag intermediate and the preparation method of Eltrombopag diethanolamine salt
ActiveCN112979481BHigh yieldHigh synthetic yieldOrganic compound preparationAmino-carboxyl compound preparationPhenylboronic acidNitration
The invention provides a preparation method of an Eltrombopag intermediate and a preparation method of Eltrombopag diethanolamine salt, which relate to the technical field of medicinal chemistry. The preparation method of the Eltrombopag intermediate uses p-bromophenol as a raw material, and the nitration reaction does not have the problem of poor selectivity. After iodination, the yield of the coupling reaction between the compound of the intermediate formula (I) and phenylboronic acid is relatively high. Subsequent reduction reaction is carried out, the debromination reaction is complete, the yield is over 90%, and the purification is relatively simple. The synthesis yield of the compound of formula (I) is high and the operation is simple. Through the above improvements, the yield of the key compound of formula (I) is high, the follow-up treatment operation is simple, and the process is easy to scale up production. The yield of the whole synthesis process of Eltrombopag diethanolamine salt is 48.1%. The operation is simple, the three wastes are less, and it is suitable for scale-up Production.
Owner:ZHEJIANG FORESTRY UNIVERSITY
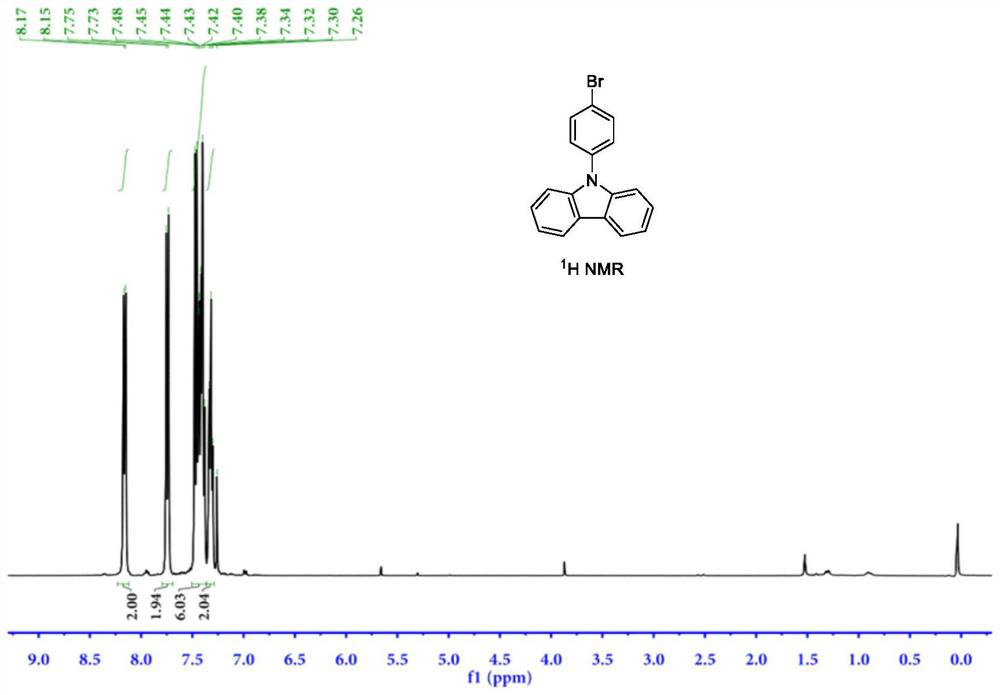
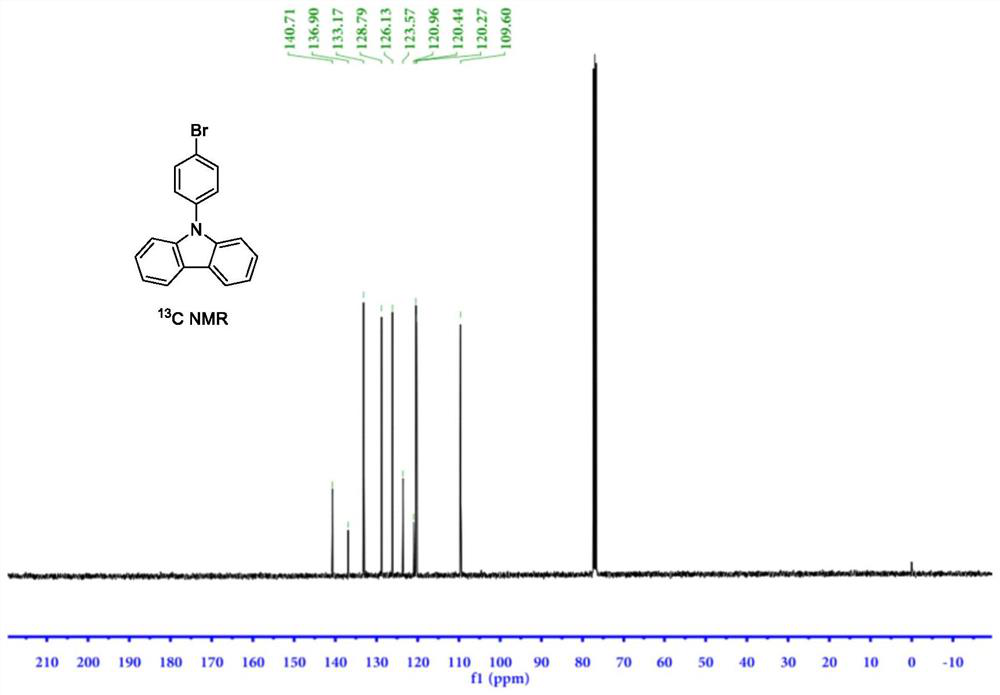
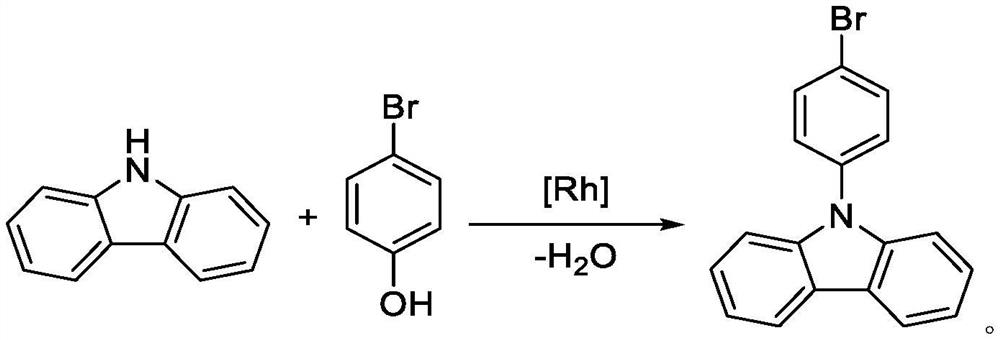
Synthesis method of 9-(4-bromophenyl) carbazole by using carbazole and p-bromophenol as raw materials
PendingCN114853658AImprove catalytic performanceIncrease profitOrganic chemistryChemical recyclingPhenyl groupPhenol
The invention discloses a synthesis method of 9-(4-bromophenyl) carbazole, and belongs to the technical field of synthesis of phenylcarbazole derivatives. The method comprises the following steps: taking carbazole and p-bromophenol as initial reactants, and synthesizing 9-(4-bromophenyl) carbazole through dehydroxylation C-N coupling reaction under the action of a catalyst, a ligand, alkali and a solvent. An imidazole N-heterocyclic carbene ligand used in the synthesis method of 9-(4-bromophenyl) carbazole is a specific type of ligand aiming at the reaction system, the ligand has strong coordination chelation and large steric hindrance, carbazole N-H and p-bromophenol can be precisely coordinated in a targeted manner when the ligand is coordinated with rhodium salt, a stable rhodium ring intermediate is generated, and the 9-(4-bromophenyl) carbazole can be used for preparing the 9-(4-bromophenyl) carbazole. Therefore, the method disclosed by the invention is mild in reaction condition, good in selectivity, simple in post-treatment, high in yield, high in atom utilization rate, environment-friendly, economical and green.
Owner:SINOSTEEL NANJING NEW MATERIALS RES INST CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com