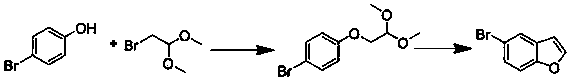
Preparation method of 5-bromobenzofuran
A furan and bromobenzene technology, applied in the field of organic synthesis, can solve the problems of expensive reagents, high cost, and limited substrates, and achieve the effects of easy industrial scale-up, reduced production costs, and avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] Add 1,4-dioxane (800 mL) into a 2000 mL three-necked round bottom flask, then add p-bromophenol (210 g, 1.213 mol), potassium carbonate (750 g, 5.426 mol) and 2- Bromoacetaldehyde dimethyl acetal (300 g, 1.775 mol). Stirring was started and heated to reflux for 24 hours. TLC and GC followed the reaction. After the reaction was complete, most of the 1,4-dioxane was distilled off. Add 600 mL each of water and ethyl acetate to the residue, stir the mixture for 30 minutes, and separate the organic phase. The aqueous phase was extracted with 200 mL of ethyl acetate, and the combined organic phases were dried overnight with sodium sulfate. The obtained crude product (340 g) was directly used in the next reaction.
[0021] Add chlorobenzene (1200 mL) to a 5000 mL three-necked round-bottomed flask, then add the above crude product 1-bromo-4'-(2,2-dimethoxyethyl)benzene (340 g), phosphoric acid (850g). Stirring was started and heated to reflux for 24 hours. TL...
Embodiment 2
[0023] Add N,N-dimethylformamide (800 mL) into a 2000 mL three-necked round bottom flask, then add p-bromophenol (210 g, 1.213 mol), potassium carbonate (750 g, 5.426 mol) and 2 -Bromoacetaldehyde dimethyl acetal (300 g, 1.775 mol). Stirring was started and heated to reflux for 20 hours. TLC and GC followed the reaction. After the reaction was completed, most of the N,N-dimethylformamide was distilled off. Add 800 mL each of water and ethyl acetate to the residue, stir the mixture for 60 minutes, and separate the organic phase. The aqueous phase was extracted twice with 300 mL of ethyl acetate. After combining the organic phases, the organic phase was washed with water (300 mL) twice and dried overnight with sodium sulfate. The obtained crude product (350 g) was directly used in the next reaction.
[0024] Add chlorobenzene (1200 mL) to a 5000 mL three-necked round-bottomed flask, then add the above crude product 1-bromo-4'-(2,2-dimethoxyethyl)benzene (350 g), phosphoric a...
Embodiment 3
[0026] Add N,N-dimethylformamide (800 mL) into a 2000 mL three-necked round bottom flask, then add p-bromophenol (210 g, 1.213 mol), potassium carbonate (750 g, 5.426 mol) and 2 - Chloroacetaldehyde dimethyl acetal (222 g, 1.78 mol). Stirring was started and heated to reflux for 28 hours. TLC and GC followed the reaction. After the reaction was completed, most of the N,N-dimethylformamide was distilled off. Add 800 mL each of water and ethyl acetate to the residue, stir the mixture for 45 minutes, and separate the organic phase. The aqueous phase was extracted twice with 300 mL of ethyl acetate. After combining the organic phases, the organic phase was washed with water (300 mL) twice and dried overnight with sodium sulfate. The obtained crude product (290 g) was directly used in the next reaction.
[0027] Add chlorobenzene (1000 mL) in a 5000 mL three-necked round-bottomed flask, then add the above crude product 1-chloro-4'-(2,2-dimethoxyethyl)benzene (290 g), phosphoric...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com