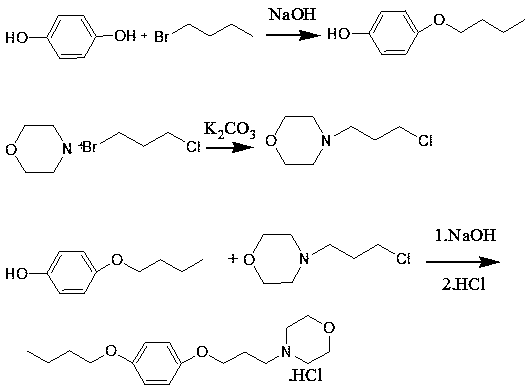
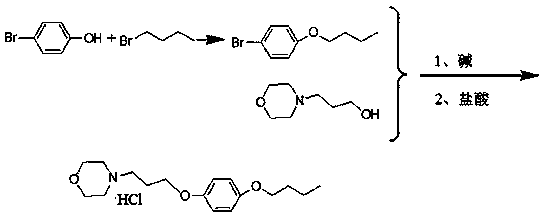
A pramoxine hydrochloride preparing method
A technology of pramoxine hydrochloride and pramoxine, which is applied in the field of pharmaceutical synthesis, can solve the problems of hydroquinone instability, no use found, and high environmental pressure, and achieve low cost, little pollution, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035] The preparation of intermediate p-bromobutyrate:
[0036] Add 17.47g (0.101mol) of p-bromophenol into a 250ml reaction flask, add 70g of water and 4.4g (0.11mol) of sodium hydroxide, stir to dissolve, add dropwise 13.7g (0.10mol) of 1-bromobutane, after the addition Heat up, reflux for 6 hours, cool down to 25°C, add 200ml of ethyl acetate, stop stirring after 10 minutes and let stand for liquid separation, wash twice with 100ml of 0.5% sodium hydroxide solution, dry with 20g of anhydrous sodium sulfate for more than 5 hours, 45 ℃ and concentrated to dryness under reduced pressure to obtain 23.2 g of a pale pink liquid with a yield of over 100 and a liquid phase purity of 98.5%.
[0037] Preparation of pramoxine hydrochloride:
[0038] Add 23.2g (0.1mol) of the above-mentioned intermediate, 17.42g (0.12mol) of 3-morpholine-1-propanol and 115g tetrahydrofuran into a 500ml reaction bottle, start stirring, slowly add 4.8g (0.12mol) of 60% sodium hydride, and control Temp...
example 2
[0040] The preparation of intermediate bromobutyrate:
[0041] Add 34.94g (0.202mol) of p-bromophenol into a 500ml reaction flask, add 140g of water and 8.8g (0.22mol) of sodium hydroxide, stir to dissolve, add dropwise 27.40g (0.20mol) of 1-bromobutane, after the addition Heat up, reflux for 6 hours, cool down to 25°C, add 200ml of ethyl acetate, stop stirring after 10 minutes, let stand for liquid separation, wash twice with 200ml of 0.5% sodium hydroxide solution, dry with 40g of anhydrous sodium sulfate for more than 5 hours, 45 ℃ and concentrated to dryness under reduced pressure to obtain 46.0 g of a pale pink liquid with a yield of over 100 and a liquid phase purity of 98.8%.
[0042] Preparation of pramoxine hydrochloride:
[0043] Add 46.0g (0.2mol) of the above-mentioned intermediate, 34.84g (0.24mol) of 3-morpholine-1-propanol and 230g tetrahydrofuran into a 1000ml reaction bottle, start stirring, slowly add 9.6g (0.24mol) of 60% sodium hydride, and control Temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com