Magnolol synthesizing method
A synthesis method and magnolol technology are applied in the field of magnolol synthesis, which can solve the problems of low magnolol yield, harsh synthesis conditions, and no industrial applicability, and achieve high yield, simple synthesis process, and low cost. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
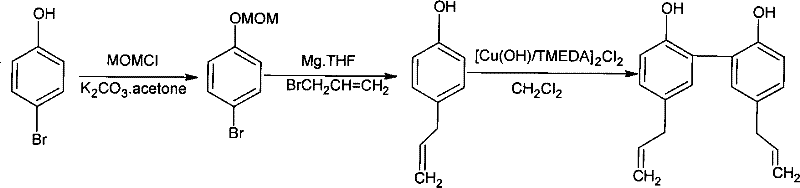
[0022] (one), figure 1 It is the synthesis route diagram of the embodiment of the present invention, such as figure 1 As shown, the whole synthesis is divided into three steps:
[0023] The first step prepares p-bromobenzyloxymethyl ether: p-bromophenol is dissolved in an acetone solvent, potassium carbonate and chloromethyl methyl ether (MOMCl) are added, and p-bromobenzyloxymethyl ether is obtained through reflux reaction;
[0024] The effect of the above reaction is to protect the hydroxyl group of p-bromophenol first, so that the hydroxyl group is not affected by the Grignard reaction described below. The expression of its reaction is as follows:
[0025]
[0026] The second step is to prepare p-allyl phenol: the p-bromobenzyloxymethyl ether prepared in the first step is reacted with allyl bromide under the participation of tetrahydrofuran solvent and magnesium chips to obtain p- Allylphenol; the expression for its reaction is as follows:
[0027]
[0028] The th...
Embodiment 1
[0037] Embodiment 1: the embodiment that the present invention synthesizes three steps, press figure 1 The synthetic route shown.
[0038] First prepare intermediate product p-allyl phenol, comprise following two steps:
[0039] Step A, prepare p-bromophenoxymethyl ether: put 25g (0.1445mol) p-bromophenol in a flask, add 200 ml acetone, dissolve p-bromophenol in acetone, then add 17.5g (0.127mol) carbonic acid Potassium, after stirring for 15 minutes, add 15g (0.185mol) chloromethyl methyl ether uniformly dropwise within about 10 minutes, reflux for 3 hours, evaporate the solvent and add water to dissolve, extract the organic phase with ether three times, add sodium hydroxide solution to adjust When the pH value was 13-14, the organic phase was washed with water until neutral, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain 28.2 g of a colorless oil, with a yield of 90%.
[0040]Step B, preparing p-allylphenol, which is divided into...
Embodiment i
[0042] Under nitrogen protection, add 5ml of anhydrous tetrahydrofuran to cover 0.24g (10mmol) of magnesium chips, add dropwise 2 drops of p-bromophenoxymethyl ether obtained in step A, add 1 grain of iodine, and stir for 5-10 minutes. See that the color fades, add 1.8g (8.29mmol) p-bromobenzyloxymethyl ether solution diluted with 10ml tetrahydrofuran dropwise evenly within 10 minutes, add 2ml tetrahydrofuran, and keep stirring at 50°C for 1 hour to obtain the obtained Add the Grignard reagent dropwise into a flask containing 1.5g (12.4mmol) allyl bromide, reflux for 4 hours, stop the reaction, cool to room temperature, add dropwise 40ml of 3mol / L hydrochloric acid solution for 2 hours, stop the reaction Separate the tetrahydrofuran phase, extract the organic phase with ether three times, wash the organic phase with sodium chloride solution until neutral, dry with anhydrous sodium sulfate, filter, and concentrate the filtrate under normal pressure to obtain a black oil, which i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com