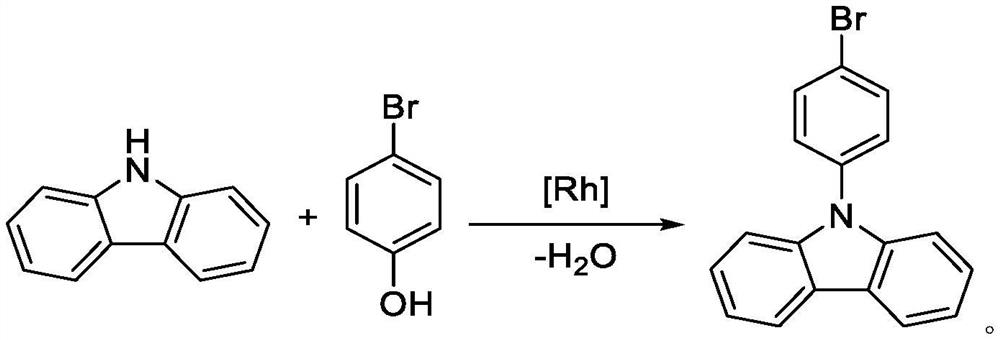
Synthesis method of 9-(4-bromophenyl) carbazole by using carbazole and p-bromophenol as raw materials
A synthesis method and technology for p-bromophenol are applied in the field of synthesis of phenylcarbazole derivatives, can solve problems such as outstanding environmental protection problems, harsh reaction conditions, poor reaction selectivity, etc., and achieve low production cost, mild reaction conditions, and strong compounding. The effect of chelation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1: a kind of synthetic method of 9-(4-bromophenyl) carbazole
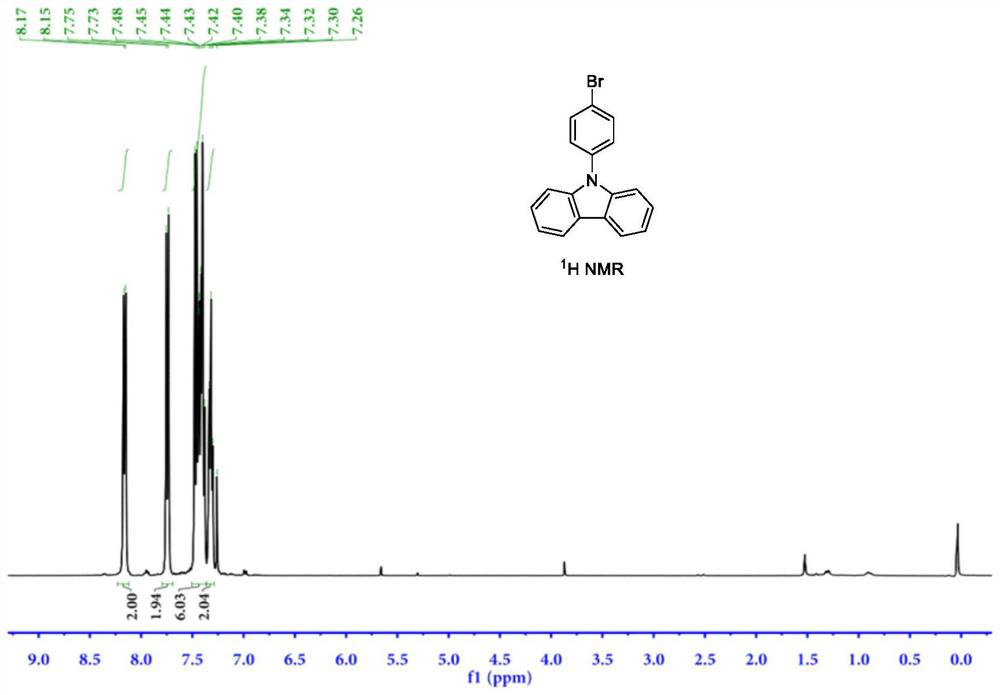
[0053] The synthesis steps include: under nitrogen protection, add 83.6g carbazole (98%, 0.5mol), 86.6g p-bromophenol (98%, 0.5mol), 1.5g [Cp*RhCl] into a 1000mL reaction flask 2 ] 2 (98%, 2.5mmol), 1.1g imidazole nitrogen heterocyclic carbene ligand NHC1 (98%, 5.0mmol), 26.5g sodium carbonate (99%, 0.25mol) and 500mL n-heptane; ℃, stirring speed 500rpm, insulation reaction 8 hours; After the reaction, cooling at room temperature filtration, washing, extraction, organic layer precipitation recovery solvent, the crude product is crystallized through n-hexane to obtain 141.2g of 9-(4-bromophenyl)carbazone azole product, the yield is 87.6%.
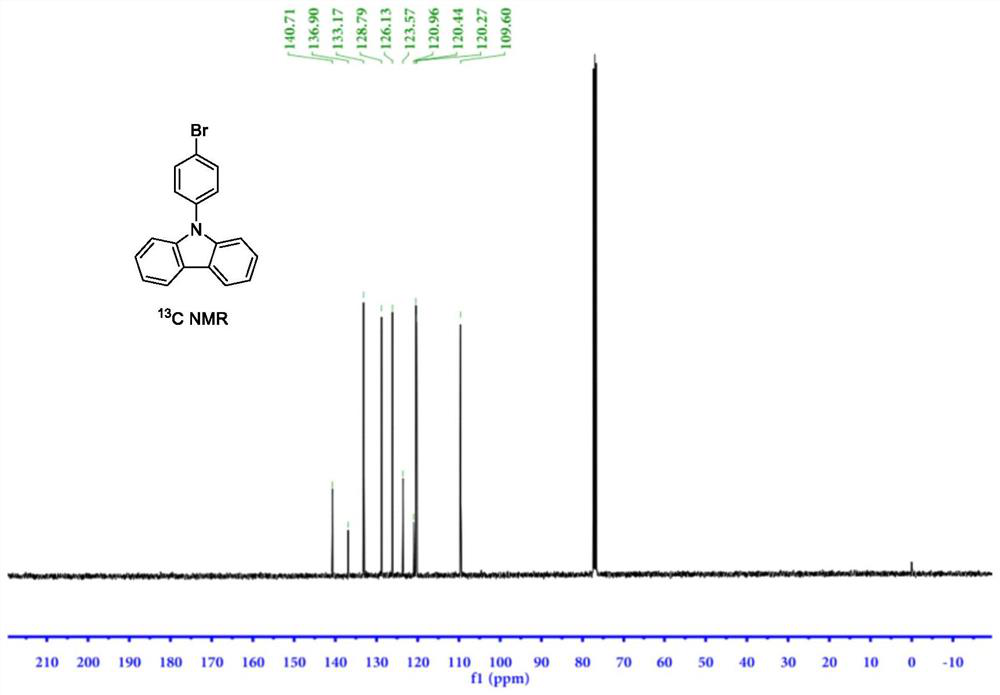
[0054] The hydrogen nuclear magnetic resonance spectrum of the product prepared in this example is as follows figure 1 shown, 1 H NMR (400MHz, CDCl 3 )d=7.32(t,J=7.2Hz,2H),7.36-7.51(m,6H),7.74(d,J=8.5Hz,2H),8.16(d,J=7.7Hz,2H); NMR carbon spectrum as figu...
Embodiment 2
[0055] Embodiment 2: a kind of synthetic method of 9-(4-bromophenyl) carbazole
[0056] The synthesis steps include: under nitrogen protection, add 83.6g carbazole (98%, 0.5mol), 86.6g p-bromophenol (98%, 0.5mol), 1.5g [Cp*RhCl] into a 1000mL reaction flask 2 ] 2 (98%, 2.5mmol), 3.8g imidazole type azacyclic carbene ligand NHC2 (98%, 12.5mmol), 41.0g sodium acetate (99%, 0.5mol) and 500mL n-octane; ℃, stirring speed 500rpm, insulation reaction 6 hours; After the reaction finishes, cooling room temperature filtration, washing, extraction, organic layer precipitation reclaims solvent, crude product is through petroleum ether crystallization, obtains 149.0g of 9-(4-bromophenyl)carbazone azole product, the yield is 92.5%.
Embodiment 3
[0057] Embodiment 3: a kind of synthetic method of 9-(4-bromophenyl) carbazole
[0058] The synthesis steps include: under nitrogen protection, add 83.6g carbazole (98%, 0.5mol), 103.9g p-bromophenol (98%, 0.6mol), 4.6gRh (PPh) into a 1000mL reaction flask 3 ) 3 Cl (98%, 5.0 mmol), 8.3 g imidazole azacyclic carbene ligand NHC 3 (98%, 25.0mmol), 26.5g of sodium carbonate (99%, 0.25mol) and 700mL of n-nonane; after feeding, the temperature was raised to 110 ° C, the stirring speed was 500 rpm, and the reaction was incubated for 8 hours; Wash with water, extract, and desolvate the organic layer to recover the solvent. The crude product is crystallized from ethanol to obtain 153.5 g of 9-(4-bromophenyl)carbazole product with a yield of 95.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com