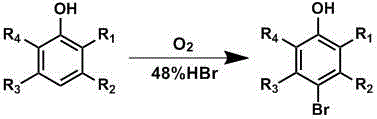
Preparation method of p-bromophenol compound
A kind of technology of phenol compound and p-bromophenol, applied in the field of preparation of p-bromophenol compound, can solve the problem of high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add manganese acetate (0.87g, 5.0mmol), 48% hydrobromic acid (12.2mL, 110mmol), phenol (9.4g, 100mmol) and n-hexanol (16.3g, 20mL) into a 250mL reactor, mix well, and close Reactor and filled with 30atm air, stirred and heated to 65 o C, after reacting for 1.5 hours, cool to room temperature, take out the mixture for analysis after degassing, and obtain remaining phenol: 2.8g, p-bromophenol: 12.1g.
Embodiment 2-3
[0020] project Catalyst (g, mmol) Phenol (g) p-Bromophenol (g) 1. 0.17,1 3.2 11.2 2. 8.7,50 1.6 14.1
Embodiment 4-8
[0022] Manganese acetate in embodiment 1 is replaced with other transition metal compounds, and the impact on reaction result is shown in the following table:
[0023] project Catalyst (g, mmol) Phenol (g) p-Bromophenol (g) 1. -- 9.2 0.2 2. Vanadium pentoxide (1.82, 10) 5.1 7.2 3. Cobalt nitrate hexahydrate (1.46, 5) 1.5 13.6 4. Iron Phthalocyanine (5.68, 10) 0 12.9 5. Ammonium chloroplatinite (0.37, 1) 9.6 0.4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com