Method for preparing 4-hydroxy-4'-cyanobiphenyl
A technology of cyanobiphenyl and hydroxyl, which is applied in the field of preparing 4-hydroxy-4'-cyanobiphenyl, can solve the problems of harsh reaction conditions, large discharge of three wastes, and low overall yield, and achieve simplification of the reaction process, The effect of large discharge of three wastes and small market capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
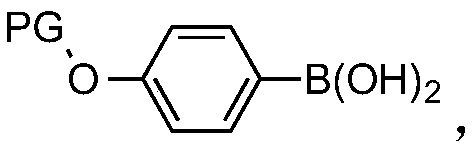
Image
Examples
Embodiment 1
[0036]Assemble a 500ml three-necked flask reaction device, put 34.6g (0.2mol) of p-bromophenol (0.2mol) and 19.75g (0.25mol) of pyridine into the three-necked flask, add 100ml of 2-methyltetrahydrofuran, control the temperature in an ice-water bath to less than 10°C, and protect with nitrogen Add 25.92g (0.24mol) of trimethylchlorosilane in 50ml of 2-methyltetrahydrofuran solution dropwise, and control the temperature not to exceed 20°C. ×2 wash the reaction liquid, and dry it with 10 g of anhydrous magnesium sulfate for later use.
[0037] Take another 1000ml three-necked flask, add 5.76g (0.24mol) of magnesium chips, put in a water bath at 30°C, add dropwise the p-trimethylsilyloxybromobenzene 2-methyltetrahydrofuran solution prepared in the previous step, and initiate the preparation of the Grignard reagent. After completion, continue to react at 30-35°C for 2 hours, cool down to -15°C in an ice-salt bath, add 100ml of 2-methyltetrahydrofuran solution of 41.6g (0.4mol) of t...
Embodiment 2
[0038] Embodiment 2 (industrialized scale)
[0039] Clean the 500L reactor device and dry it. Put 34.6kg of p-bromophenol and 20.19kg of dihydropyran into the reactor, add 100L of xylene, control the temperature of the freezing liquid below -10°C, and add three 25.92kg of methyl chlorosilane in 50L xylene solution, the temperature control does not exceed 0°C, after dropping, the temperature of the cooling liquid temperature control system is -5~5°C, the reaction is terminated after 4 hours of reaction, and the reaction solution is washed with 50L×2 saturated saline, 10kg of anhydrous magnesium sulfate was dried for later use.
[0040] In another dry 1000L reaction kettle, add 5.76kg of magnesium chips, heat to 30°C, add dropwise the p-trimethylsiloxy bromoxylene solution prepared in the previous step, trigger the preparation of Grignard reagent, and continue to control React at 30-35°C for 4 hours, cool the freezing liquid to -10°C, add 100L xylene solution of 41.6kg of trime...
Embodiment 3
[0041] Embodiment 3 (industrialized scale)
[0042] Clean and dry the 500L reactor device, put 34.6kg of p-bromophenol and 17kg of imidazole in the reactor, add 100L of dioxane, control the temperature of the freezing liquid below 20°C, and add triisopropyl group dropwise under nitrogen protection Chlorosilane 46.08kg in 50L dioxane solution, the temperature control does not exceed 30°C, after dropping, the water bath temperature is controlled at 15-25°C, the reaction is terminated after 4 hours of reaction, the reaction solution is washed with saturated saline 50L×2, and 10kg of anhydrous Magnesium sulfate is dried for later use.
[0043] In another dry 1000L reaction kettle, add 6kg of magnesium chips, heat to 40°C, add dropwise the prepared p-triisopropylsilyloxybromobenzenedioxane solution to initiate the preparation of Grignard reagent. Continue to control the temperature at 50-60°C for 4 hours, cool down the cooling liquid to 20°C, add 45.12 kg of triisopropyl borate in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com