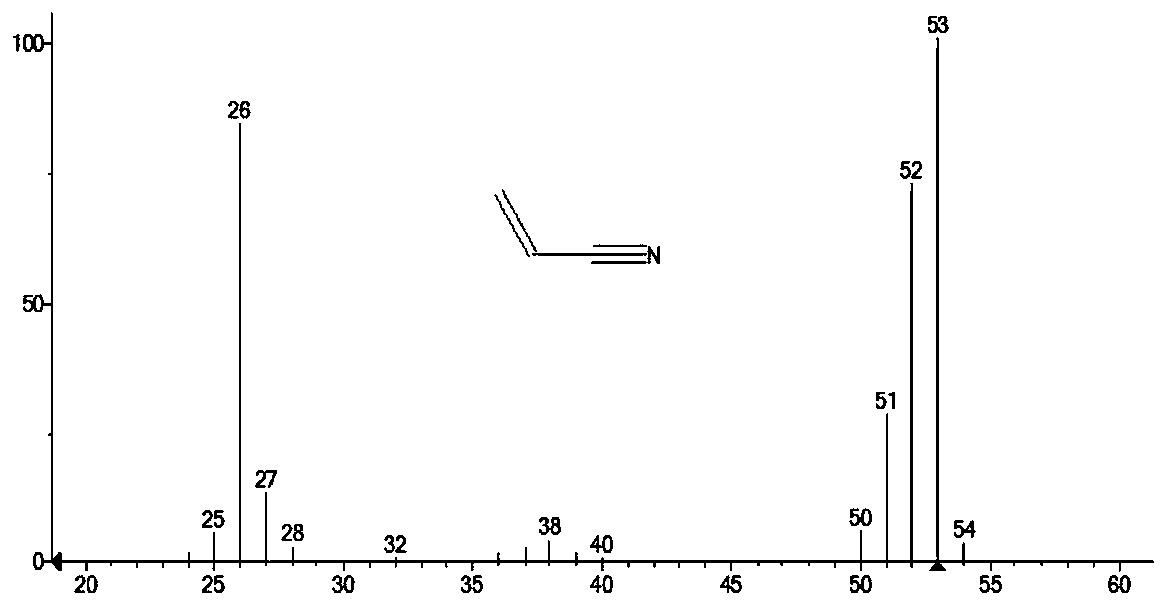
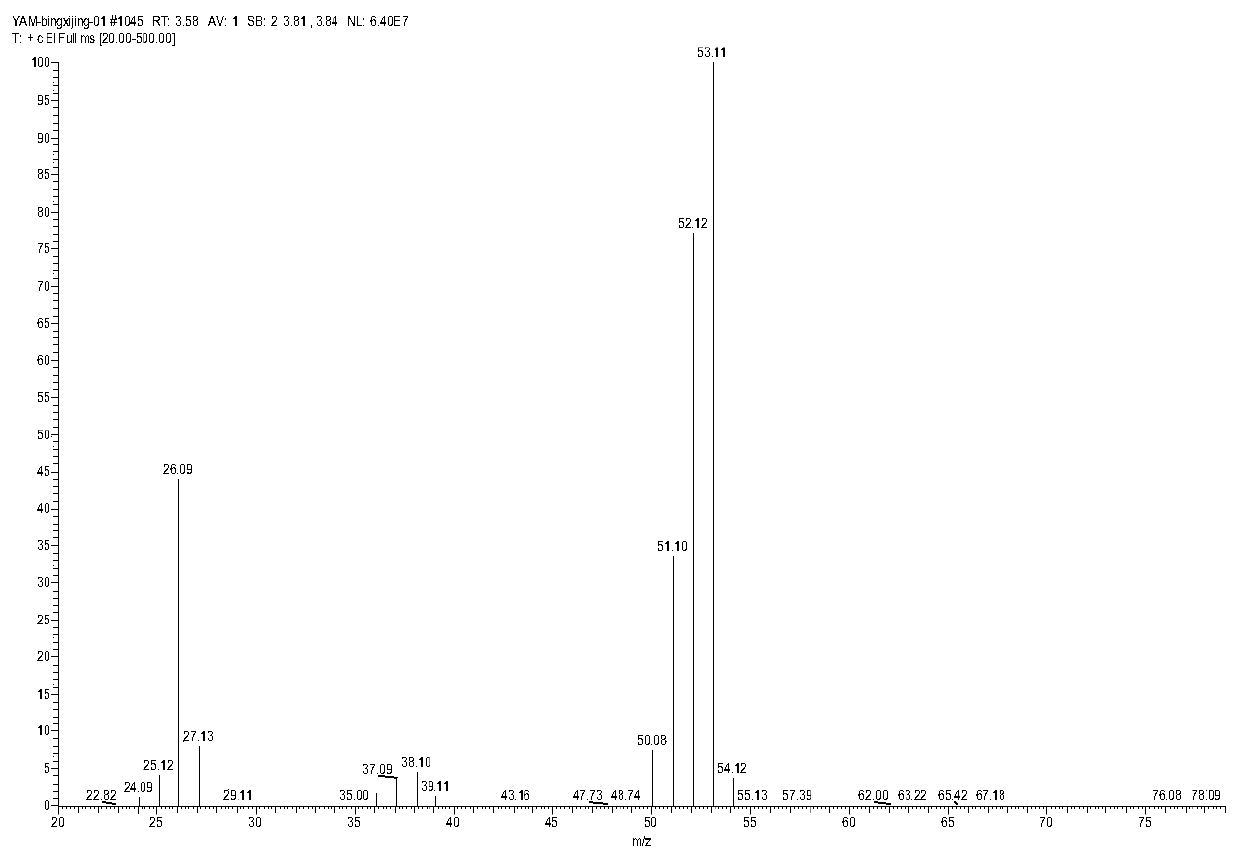
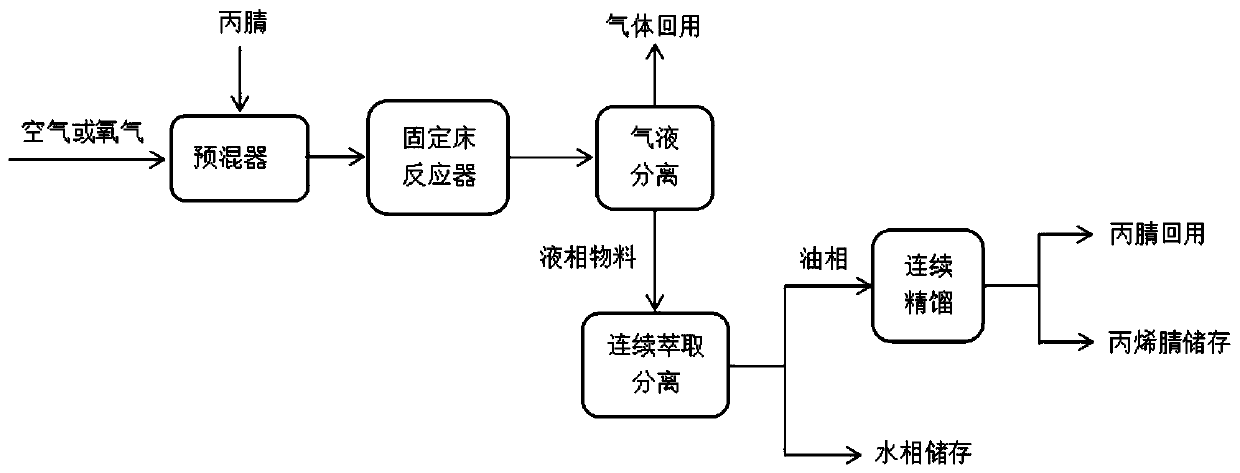
Method for preparing acrylonitrile by continuous oxidative dehydrogenation
A technology for oxidative dehydrogenation and acrylonitrile, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitrile, etc., can solve the problems of large pollution and energy consumption, achieve high yield, short separation process, The effect of small market capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Weigh 30.00g of nickel nitrate hexahydrate and add it to 80mL of cobalt nitrate hexahydrate solution with a concentration of 0.5mol / L, stir to dissolve it, then weigh 10.00g of ammonium molybdate, dissolve it in the above solution, and then add 2.5mol Add / L NaOH solution to the above solution, adjust the pH to 7, stir for 30min, then add 7.5g of zirconium hydroxide, and continue stirring for 1.0h. Then the mixed solution was transferred into a polytetrafluoroethylene reactor, and the reactor was placed in a blast drying oven at 160° C. for 12 hours. Take out the reaction kettle, cool it to room temperature, use a vacuum pump; vacuum filter the filter bottle and filter paper, wash the organic solvent repeatedly with absolute ethanol and then wash it with distilled water, put it in an oven and dry it for 12 hours at 80°C to obtain a catalyst sample, and then A catalyst for a fixed bed is prepared by a catalyst shaping machine.
Embodiment 2
[0066] Weigh 48.40g nickel nitrate hexahydrate and add it to 80mL of cobalt nitrate hexahydrate solution with a concentration of 0.5mol / L, stir to dissolve it, then weigh 14.85g ammonium molybdate, dissolve it in the above solution, and then add 2.5mol Add / L NaOH solution to the above solution, adjust the pH to 7, stir for 30min, then add 11.38g of zirconium hydroxide, and continue stirring for 1.0h. Then the mixed solution was transferred into a polytetrafluoroethylene reactor, and the reactor was placed in a blast drying oven at 160° C. for 12 hours. Take out the reaction kettle, cool it to room temperature, use a vacuum pump; vacuum filter the filter bottle and filter paper, wash the organic solvent repeatedly with absolute ethanol and then wash it with distilled water, put it in an oven and dry it for 12 hours at 80°C to obtain a catalyst sample, and then A catalyst for a fixed bed is prepared by a catalyst shaping machine.
Embodiment 3
[0068] Weigh 52.85 nickel nitrate hexahydrate and add it to 80 mL of cobalt nitrate hexahydrate solution with a concentration of 0.5 mol / L, stir to dissolve it, then weigh 14.85 and dissolve it in the above solution, then add 2.5 mol / L NaOH solution to In the above solution, adjust the pH to 7, stir for 30 min, then add 7.58 g of zirconium hydroxide, and continue stirring for 1.0 h. Then transfer the mixed solution into the polytetrafluoroethylene reactor, and put the reactor into Constant 12h in the blast drying oven. Take out the reaction kettle, cool it to room temperature, use a vacuum pump; vacuum filter the filter bottle and filter paper, wash the organic solvent repeatedly with absolute ethanol and then wash it with distilled water, put it in an oven and dry it for 12 hours at 80°C to obtain a catalyst sample, and then A catalyst for a fixed bed is prepared by a catalyst molding machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com