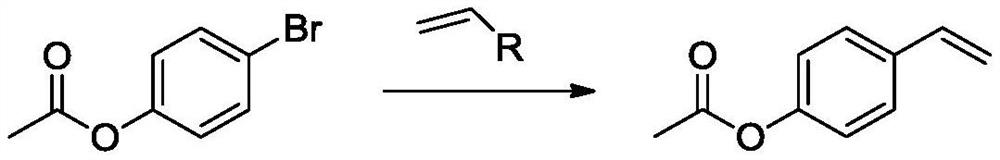
Synthetic method of p-acetoxystyrene
A technology of acetoxystyrene and acetoxybromobenzene is applied in the field of synthesis of p-acetoxystyrene, can solve the problems of low yield, difficult availability of reaction raw material p-acetoxyphenethyl alcohol, etc. High efficiency, mild reaction conditions and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
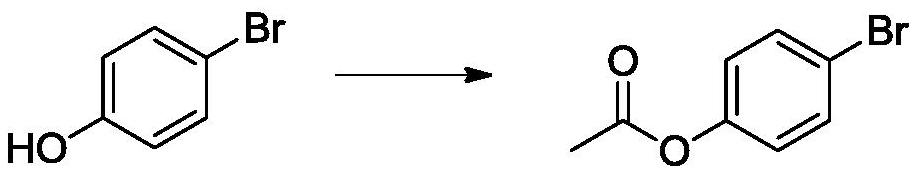
[0038]In a 1L reaction flask, add 450g ethyl acetate, p-bromophenol (86.5g, 0.5mol), and sodium carbonate (63.6g, 0.6mol) in sequence. Turn on the stirring, keep the temperature at 0°C, and add acetyl chloride (58.8 g, 0.75 mol) dropwise. After the dripping is completed, continue to heat and stir for 8 hours, wash 3 times with water, stand for separation, and distill the organic layer to recover the solvent to obtain 98 g of light brown liquid, namely 4-acetoxybromobenzene, with a yield of 91%. The purity is 96.0%.
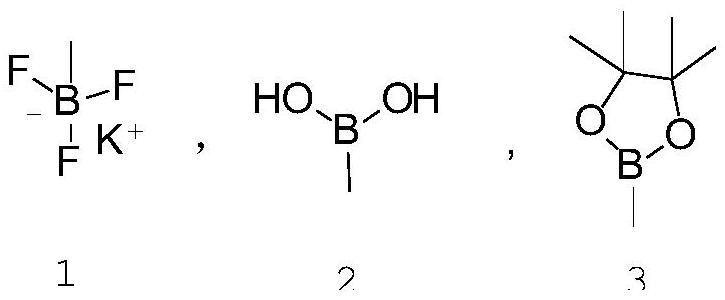
[0039]Add 4-acetoxybromobenzene (107.5g, 0.5mol), vinylboronic acid (39.5g, 0.55mol), palladium tetrakistriphenylphosphine (2.9g, 2.5mmol), sodium phosphate (163.9g) into the reaction flask , 1.0mol), ethanol (500mL), mix and stir. Then, the reaction was carried out at 100°C for 12 hours. After the reaction is completed, cool to room temperature, wash with water and stand for separation. The organic phase is dried over anhydrous magnesium sulfate overnight. After filtratio...
Embodiment 2
[0042]In a 2L reaction flask, add 600g of dichloromethane, p-bromophenol (86.5g, 0.5mol), and triethylamine (65.7g, 0.65mol) in sequence. Turn on the stirring, keep the temperature at 10°C, and add acetyl chloride (51 g, 0.65 mol) dropwise. After the dropwise addition is completed, after stirring for 6 hours at room temperature, wash with 10% sodium bicarbonate aqueous solution for 3 times, stand for layering, separate the organic phase, dry over anhydrous magnesium sulfate overnight, filter the organic layer and distill the solvent to obtain 101g The light brown liquid is 4-acetoxybromobenzene with a yield of 94% and a purity of 96.5%.
[0043]Add 4-acetoxy bromobenzene (107.5g, 0.5mol), vinyl borate (101.4g, 0.6mol), palladium acetate (0.9g, 4mmol), potassium carbonate (82.9g, 0.6mol) into the reaction flask ), dichloroethane (500 mL). Mix and stir, and then react for 24 hours at 80°C. After the reaction is completed, it is cooled to room temperature, washed with water and left for s...
Embodiment 3
[0045]In a 500mL reaction flask, p-bromophenol (86.5g, 0.5mol) and acetic anhydride (61.2g, 0.6mol) were sequentially added, and the reaction was stirred for 2h while maintaining the temperature at 180°C. After the completion of the reaction, vacuum distillation was performed to obtain 102 g of a reddish brown oily liquid, namely 4-acetoxybromobenzene, with a yield of 95% and a purity of 95.8%.
[0046]Add 4-acetoxybromobenzene (107.5g, 0.5mol), potassium ethylene trifluoroborate (87.1g, 0.65mol) and [1,1'-bis(diphenylphosphino)ferrocene into the reaction flask ] Palladium dichloride (3.9g, 5mmol), sodium carbonate (127.2g, 1.2mol), dimethylformamide (500mL), mixed and stirred, and then reacted at 100°C for 8 hours. After the reaction is completed, cool to room temperature, wash with water and stand for separation. After the organic phase is dried over anhydrous magnesium sulfate, it is distilled to obtain 74.2g of colorless liquid, namely p-acetoxystyrene, with a yield of 91% and a pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com