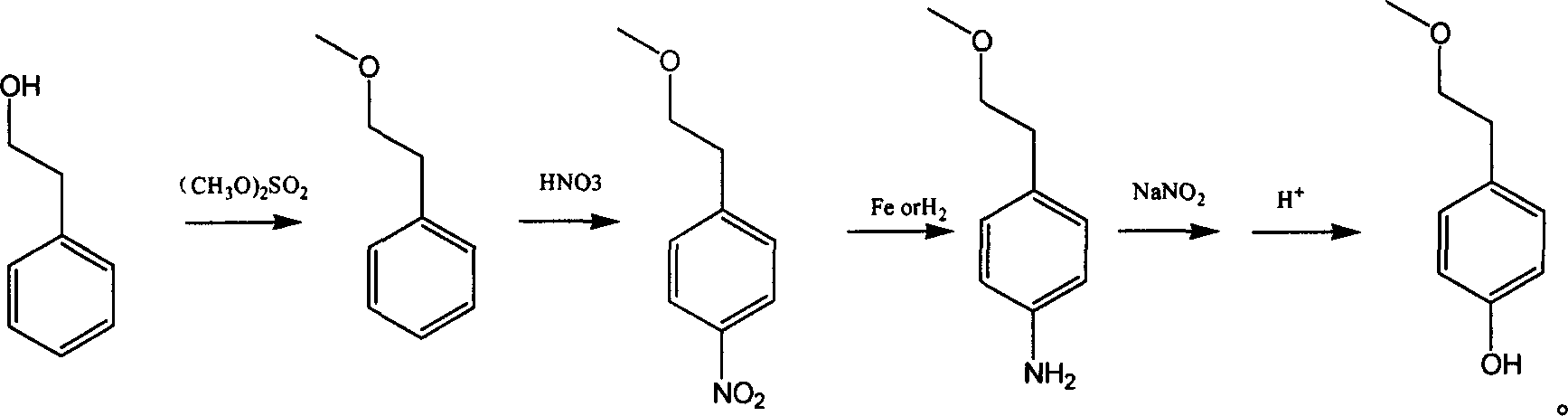
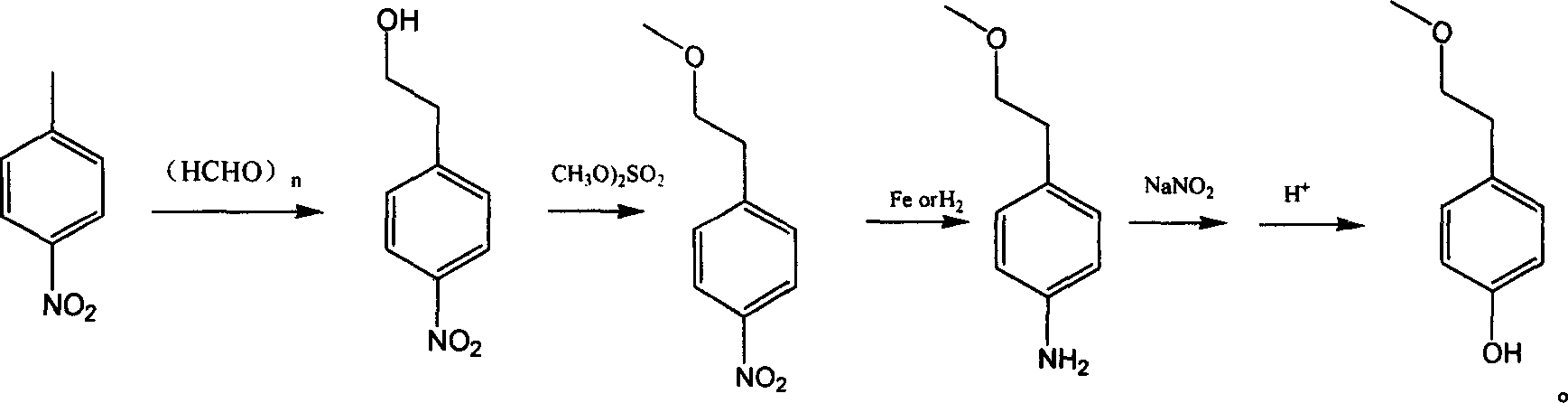
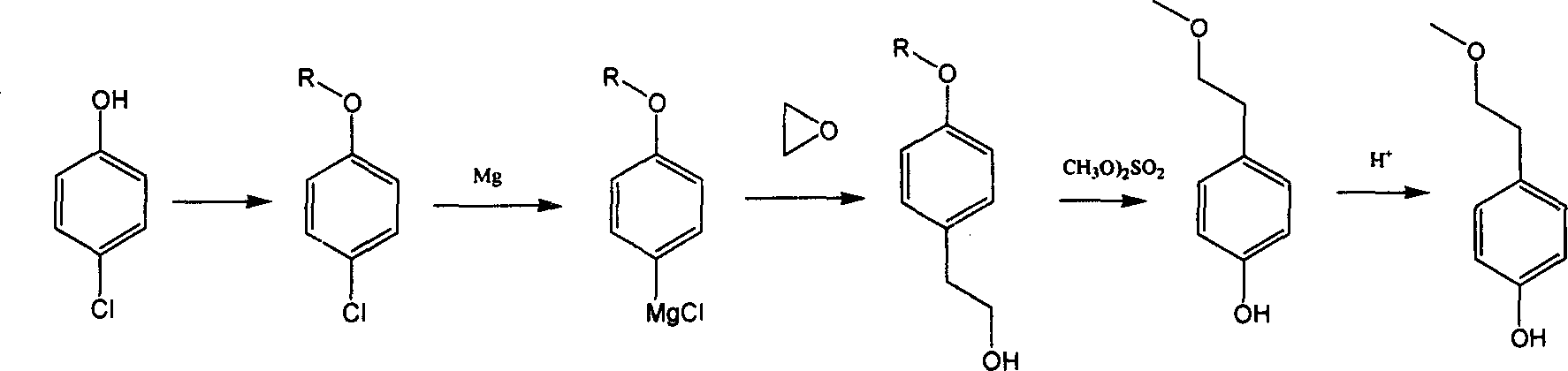
Para-(2-methoxyl) ethylphenol synthesis method
A technology of ethyl phenol and synthesis method, which is applied in the direction of preparation of ether from alkylene oxide, preparation of ether, organic chemistry, etc. It can solve the problems of poor synthesis selectivity and large production pollution, and achieve less three wastes, high product yield and stable product good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 128 grams (1.0mol) of p-chlorophenol, 500ml of toluene, and 2 grams of concentrated sulfuric acid in a 1000ml three-necked flask, and pass through isobutylene under water bath cooling conditions to react until more than 90% of p-chlorophenol is converted into p-chlorophenyl tert-butyl After ether, the isobutene was stopped. Cool to a lower temperature and terminate the reaction with 30% sodium hydroxide aqueous solution and wash to remove unreacted p-chlorophenol, and then wash with water until nearly neutral. The obtained p-chlorophenyl tert-butyl ether toluene solution was concentrated to obtain about 420 grams of toluene solution containing about 40% p-chlorophenyl tert-butyl ether for the next reaction.
Embodiment 2
[0021] Add 172 grams (1.0mol) of p-bromophenol, 500ml of toluene, and 2 grams of concentrated sulfuric acid in a 1000ml three-necked flask, and pass through isobutylene under water bath cooling conditions to react until more than 90% of p-chlorophenol is converted into p-chlorophenyl tert-butyl After ether, the isobutene was stopped. Cool to a lower temperature and terminate the reaction with 30% sodium hydroxide aqueous solution and wash to remove unreacted p-bromophenol, and then wash with water until nearly neutral. The obtained p-chlorophenyl tert-butyl ether toluene solution was concentrated to obtain about 420 grams of toluene solution containing about 40% p-chlorophenyl tert-butyl ether for the next reaction.
Embodiment 3
[0023] Add 500ml tetrahydrofuran, 24 grams (1.0mol) magnesium in 2000ml there-necked flask, add about 5-10% of the reaction solution of the previous step under reflux conditions to initiate the reaction, and then control the dropwise addition of the parachlorine obtained in the step reaction under reflux conditions. After the dropwise addition of the phenyl tert-butyl ether toluene solution is completed, it is refluxed until the p-chlorophenyl tert-butyl ether reacts substantially completely to obtain a Grignard reaction solution. Then, under cooling conditions, 48.4 grams (1.1 mol) of ethylene oxide was slowly added to react to obtain p-tert-butoxyphenethyl alcohol. The reaction liquid was washed with water, separated into layers to obtain an organic phase, and the organic phase was concentrated and rectified to obtain about 125 grams of p-tert-butylphenylethanol (purity>95%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com