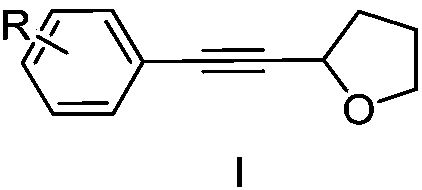
Method for synthesizing alpha-alkynyl substituted ether compounds
A technology to replace ethers and compounds, applied in the field of organic synthetic chemistry, can solve the problems of complex synthesis of hypervalent iodine, achieve good compatibility, simple and easy synthesis method, and wide range of biological activities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0026] Synthesis of 2-(phenylethynyl)tetrahydrofuran
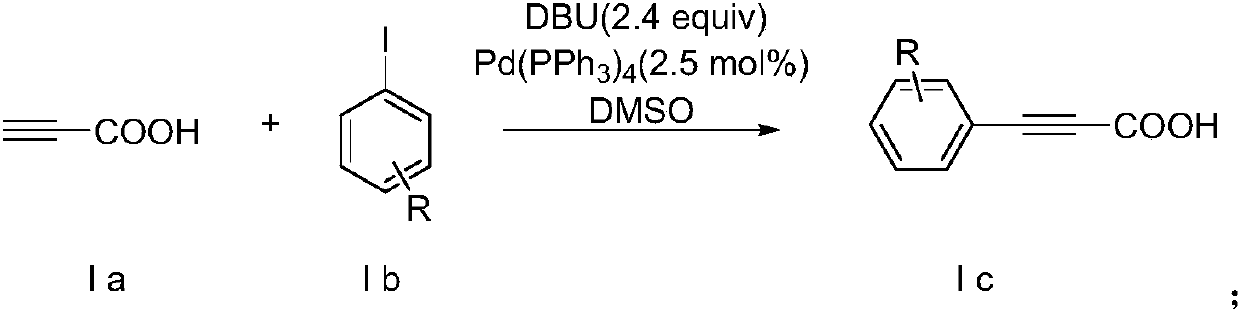
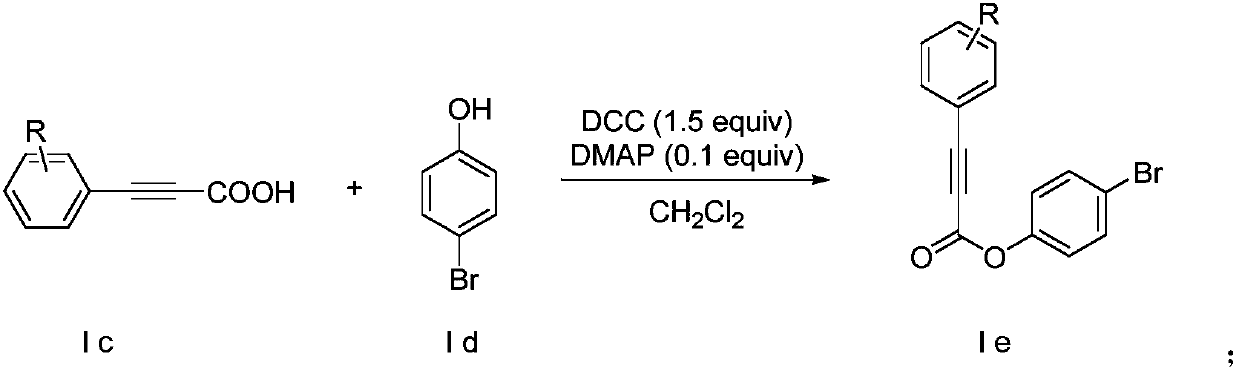
[0027] In a reaction vessel, iodobenzene (10.0mmol), DBU (3.66g, 24mmol, 2.4equiv), Pd(PPh 4 ) 3 (288mg, 0.26mmol, 2.5mol%) was dissolved in 12ml of DMSO to form solution a, then propiolic acid (840mg, 12mmol, 1.2equiv) was dissolved in 12ml of DMSO to form solution b, and finally solution b was slowly was added dropwise to solution a, stirred and reacted at room temperature for 12 hours. After the reaction, add 25ml of ethyl acetate to the reaction solution, extract with saturated sodium bicarbonate solution, adjust the pH of the collected water layer to 2.0 with 1mol / L hydrochloric acid, and finally extract the organic matter with dichloromethane. layer, separated the organic phase and used anhydrous sodium sulfate to remove water, and after drying, removed the solvent by distillation under reduced pressure to obtain a crude product, and separated the crude product by column chromatography to obtain a phenylpropiolic a...
experiment example 2
[0030] Synthesis of 2-((4-chlorophenyl)ethynyl)tetrahydrofuran
[0031] In a reaction vessel, p-chloroiodobenzene (10.0mmol), DBU (3.66g, 24mmol, 2.4equiv), Pd(PPh 4 )3 (288mg, 0.26mmol, 2.5mol%) was dissolved in 12ml of DMSO to form solution a, then propiolic acid (840mg, 12mmol, 1.2equiv) was dissolved in 12ml of DMSO to form solution b, and finally solution b was slowly was added dropwise to solution a, stirred and reacted at room temperature for 12 hours. After the reaction, add 25ml of ethyl acetate to the reaction solution, extract with saturated sodium bicarbonate solution, adjust the pH of the collected water layer to 2.0 with 1mol / L hydrochloric acid, and finally extract the organic matter with dichloromethane. layer, separated the organic phase and used anhydrous sodium sulfate to remove water, and after drying, used vacuum distillation to remove the solvent to obtain a crude product, and separated the crude product by column chromatography to obtain p-chlorophenylp...
experiment example 3
[0034] Synthesis of 2-((4-methylphenyl)ethynyl)tetrahydrofuran
[0035] In a reaction vessel, p-methyliodobenzene (10.0mmol), DBU (3.66g, 24mmol, 2.4equiv), Pd(PPh 4 ) 3 (288mg, 0.26mmol, 2.5mol%) was dissolved in 12ml of DMSO to form solution a, then propiolic acid (840mg, 12mmol, 1.2equiv) was dissolved in 12ml of DMSO to form solution b, and finally solution b was slowly was added dropwise to solution a, stirred and reacted at room temperature for 12 hours. After the reaction, add 25ml of ethyl acetate to the reaction solution, extract with saturated sodium bicarbonate solution, adjust the pH of the collected water layer to 2.0 with 1mol / L hydrochloric acid, and finally extract the organic matter with dichloromethane. layer, separated the organic phase and used anhydrous sodium sulfate to remove water, and after drying, used vacuum distillation to remove the solvent to obtain a crude product, and separated the crude product by column chromatography to obtain p-tolylpropio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com