A novel sulfonyl chloride derivative, its preparation method and its application
A technology for sulfonyl chloride derivatives and compounds, applied in the field of sulfonyl chloride derivatives, can solve problems such as unfavorable synthesis of derivatives, and achieve the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
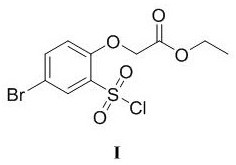
[0036] Example 1 Preparation of ethyl 2-(4-bromo-2-(chlorosulfonyl)phenoxy)acetate (compound I)
[0037] Step 1 Synthesis of 2-(4-bromophenoxy) ethyl acetate
[0038]
[0039] Compound 1, i.e. 4-bromophenol (3g, 17.34mmol) was dissolved in 10mL of DMF, followed by adding ethyl chloroacetate (2.55g, 20.81mmol) and potassium carbonate (7.18g, 52.02mmol), and at 80 Stir at ℃ for 3-4h.
[0040] After the reaction was complete, water was added to the mixture and stirred. The precipitated solid was suction-filtered to obtain 4.46 g of the target compound as a white solid, yield: 99.3%, and this white solid was ethyl 2-(4-bromophenoxy)acetate (compound 2).
[0041] 1 H NMR (400MHz, CDCl 3 )δ7.39(d, J=9.0Hz, 2H), 6.80(d, J=9.0Hz, 2H), 4.59(s, 2H), 4.27(q, J=7.0Hz, 2H), 1.30(t, J=7.3Hz,3H).MS(ESI)m / z calcd for C 10 h 11 BrO 3 257.98&259.98;found[M+Na] + 283.3.
[0042] Step 2 Synthesis of ethyl 2-(4-bromo-2-(chlorosulfonyl)phenoxy)acetate
[0043]
[0044] Compound 2, ...
Embodiment 2
[0047] The preparation process optimization of embodiment 2 compound I
[0048] (1) Exploration of preliminary synthesis conditions
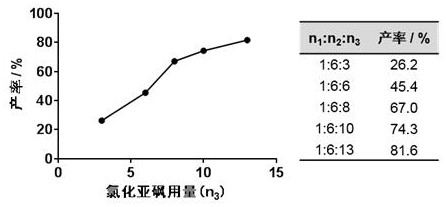
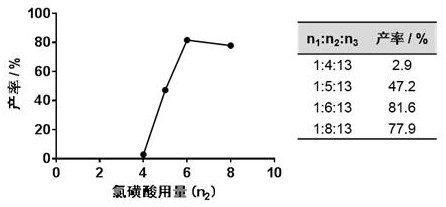
[0049] In the field, there is currently no report on the synthesis of compound I. The present invention intends to prepare compound I by direct chlorosulfonation of chlorosulfonic acid. Preliminary studies have found that the target compound cannot be obtained by adding chlorosulfonic acid alone (Table 1, experiments 1-3). After analysis, the present invention adds thionyl chloride to the reaction system to finally obtain the target product.
[0050] It is worth noting that when using different concentrations of thionyl chloride to participate in the reaction, the reaction yield of the low-concentration thionyl chloride solution was significantly lower than that of the high-concentration thionyl chloride (Table 1, experiments No. 4 to 5), It shows that the concentration and dosage of thionyl chloride have great influence on the reaction yield...
Embodiment 3
[0065] Application of Example 3 Compound I in the Preparation of Sulfonamide, Biphenyl and Amide Derivatives
[0066] The application of compound I in the preparation of sulfonamide, biphenyl and amide derivatives is as follows: Figure 5 shown.
[0067] Here are a few applications:
[0068] (1) Compound I is used to prepare sulfonamide derivatives
[0069]
[0070] Dissolve ethyl 2-(4-bromo-2-(chlorosulfonyl)phenoxy)acetate (100mg, 0.28mmol) and n-propylamine (24.8mg, 0.42mmol) in 6mL of dichloromethane, add 0.5mL of pyridine, 40°C The reaction was carried out for 1.5 h, and the reaction was monitored by TLC. After the reaction, add 20mL of water and 3-5mL of dilute hydrochloric acid, extract with ethyl acetate (15mL×2), wash the organic layer once with water, once with saturated brine, dry over anhydrous sodium sulfate, concentrate under reduced pressure, and pass the crude product through Separation by silica gel column chromatography gave the target compound ethyl 2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com