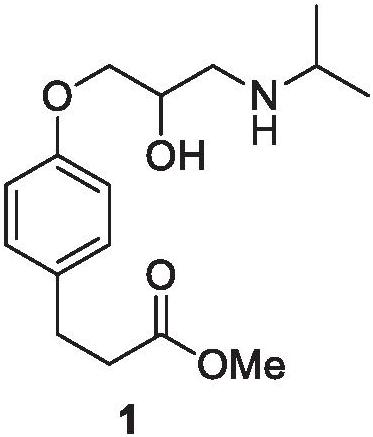
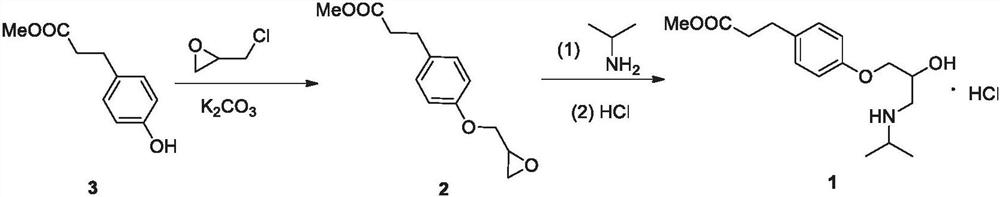
Preparation method of esmolol hydrochloride intermediate
A technology for esmolol hydrochloride and intermediates, which is applied in the field of preparation of esmolol hydrochloride intermediate 4-hydroxyphenylpropionate methyl ester, which can solve the problem of high operation requirements, long reaction steps, and poor atom economy and other problems, to achieve the effects of short preparation steps, mild reaction conditions, and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
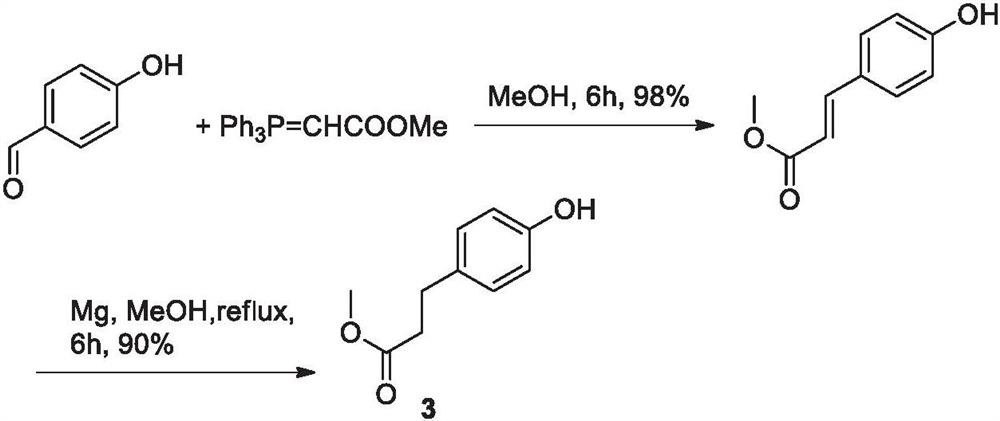
Embodiment 1
[0042] Synthesis of step (a) 3-(4-hydroxyphenyl) methyl acrylate
[0043] Dissolve 17.3g (100mmol) p-bromophenol in 258g dioxane, add 0.224g palladium acetate (1mmol), 0.787g triphenylphosphine (3mmol), 11.865g pyridine (150mmol), and finally add 10.33g methyl acrylate (120mmol), heated to 80°C under the protection of nitrogen, cooled to room temperature after 10h of reaction, distilled off the solvent under reduced pressure, then added 100ml of a mixed solvent of ethyl acetate and n-hexane, washed with water and brine and dried, added activated carbon, filtered and evaporated to dryness The solvent obtained 18.1 g of an oily product, the HPLC purity of the addition of cis and trans olefin products was 91%, and it was directly put into the next step without purification.
[0044] The preparation of step (b) methyl 4-hydroxyphenylpropionate (3)
[0045] Add the crude 3-(4-hydroxyphenyl)methyl acrylate obtained in the previous step to 450 g of ethanol, add 2% target carbon (bas...
Embodiment 2
[0047] Synthesis of step (a) 3-(4-hydroxyphenyl) methyl acrylate
[0048] Dissolve 17.3g (100mmol) p-bromophenol in 258g DMF, add 0.177g palladium chloride (1mmol), 0.787g triphenylphosphine (3mmol), 15.2g triethylamine (150mmol), and finally add 10.33g methyl acrylate (120mmol), heated to 70°C under the protection of nitrogen, cooled to room temperature after 15h of reaction, distilled off the solvent under reduced pressure, then added 100ml of a mixed solvent of ethyl acetate and n-hexane, washed with water and brine and dried, added activated carbon, filtered and evaporated to dryness The solvent obtained 17.5 g of an oily substance, the HPLC purity of the addition of cis and trans olefins was 89%, and it was directly put into the next step without purification.
[0049] Step (b) Preparation of 4-(2-methoxyethyl)phenol (3)
[0050] The 3-(4-hydroxyphenyl) methyl acrylate crude product obtained by the previous step reaction is added to 375g of dioxane, 2% target carbon (based...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com