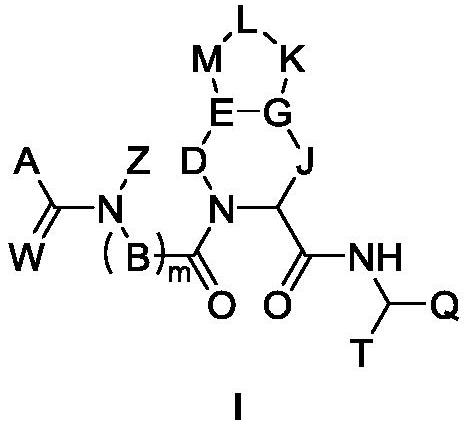
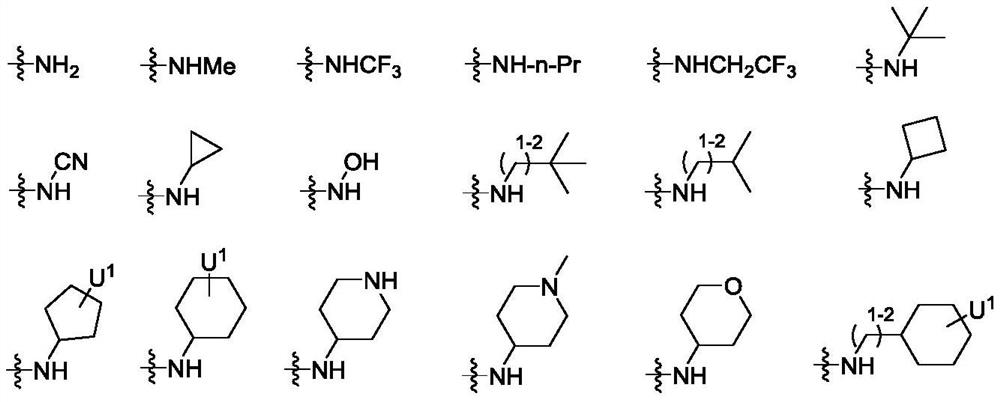
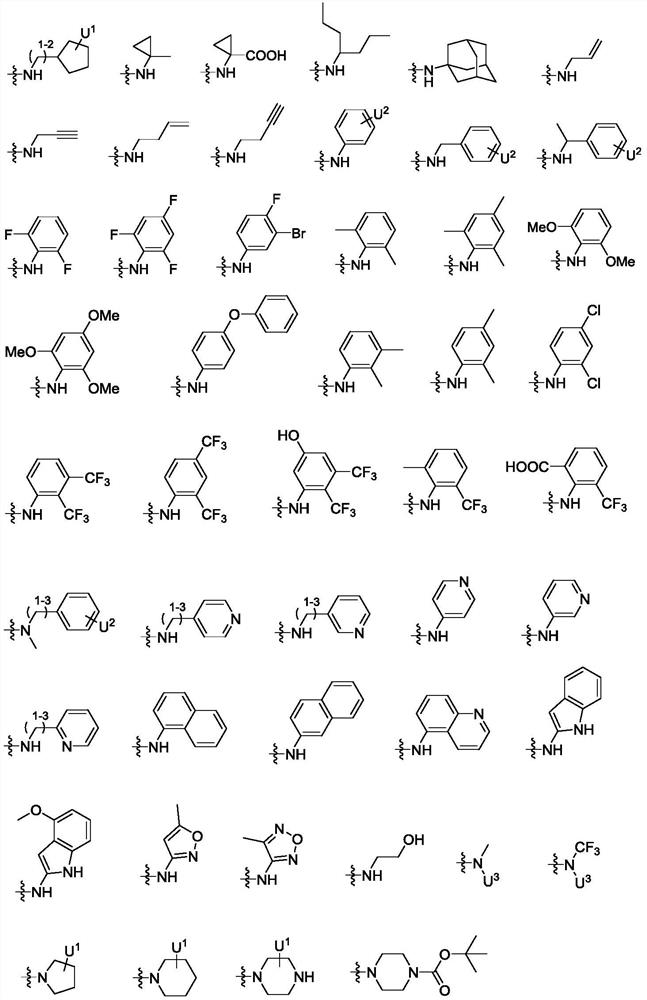
Peptidomimetic compound as well as derivative, preparation method, pharmaceutical composition and application thereof
A technology of compounds and derivatives, which is applied in the field of peptidomimetic compounds and their derivatives, can solve the problems of inability to respond to late treatment of patients with viral infections in a timely and effective manner, threaten the lives of patients, and have toxic and side effects, and achieve easy operation and applicability Broad and efficient inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Embodiment 1: synthetic compound S1
[0154] (1R,2S,5S)-3-((S)-2-(3-(tert-butyl)ureido)-3,3-dimethylbutyryl)-N-((S)-1-cyano Base-2-((S)-2-oxopyrrolidin-3-yl)ethyl)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide (S1 )
[0155]
[0156]
[0157] Step 1: Synthesis of compound 2
[0158] Weigh raw material 1 (2g, 10mmol), dissolve N-Boc-L-tert-leucine (2.3g, 10mmol) in N,N-dimethylformamide (20mL), add N,N, N',N'-tetramethyl-O-(7-azabenzotriazol-1-yl)urea hexafluorophosphate (3.7 g, 12 mmol) and N-methylmorpholine (3.3 mL, 30 mmol), Maintain 0 ℃ reaction 3h. Add saturated ammonium chloride solution (20mL) to quench, extract with ethyl acetate (30mL×3), wash with saturated brine (30mL), dry over anhydrous sodium sulfate, filter, concentrate, make sand, and purify by column chromatography (PE : EA=10:1) to obtain white foamy solid compound 2 (2.8 g, 75%). 1 H NMR (400MHz, Chloroform-d) δ5.11(d, J=10.2Hz, 1H), 4.46(s, 1H), 4.20(d, J=10.3Hz, 1H), 3.99(d, J=10....
Embodiment 2
[0219] Embodiment 2: synthetic compound S39
[0220]
[0221] Step 1: Synthesis of Compound 13
[0222] Weighed triphosgene (930mg, 3.1mmol) and dissolved it in anhydrous dichloromethane (5mL), added raw material 3 (1g, 3.1mmol) at 0°C under nitrogen protection, stirred for 5min, and slowly added triethylamine ( 0.9mL, 6.2mmol), stirred at room temperature for 2h, moved to 0°C and slowly added isobutanol (0.6mL, 6.2mmol), and reacted overnight at room temperature. Stop the reaction, add saturated ammonium chloride solution (10 mL) to quench, diatomaceous earth filter, remove the solvent under reduced pressure, extract with dichloromethane (10 mL), wash with saturated brine (10 mL), dry over anhydrous sodium sulfate, filter, Concentrate, make sand, and purify by column chromatography (DCM:MeOH=20:1) to obtain compound 13 (734 mg, 62%) as a colorless liquid. 1 H NMR (300 MHz, Chloroform-d) δ4.68–4.62(m,1H),4.24(d,J=4.8Hz,1H),4.17(d,J=8.1Hz,1H), 3.87(s,3H ),3.54(dd,J=12.6,4...
Embodiment 3
[0243] Embodiment 3: synthetic compound S173
[0244]
[0245] Step 1: Synthesis of compound 15
[0246] Compound 8 (2.0 g, 7.0 mmol) was weighed and dissolved in dioxane solution, and a solution of hydrogen chloride in dioxane (17.6 mL, 70 mmol) was slowly added dropwise at 0° C., and stirred at room temperature for 6 h. The reaction was stopped, and the solvent was removed under reduced pressure to obtain a white solid, which was directly used in the next reaction.
[0247] Step 2: Synthesis of compound 16
[0248] Dissolve compound 5 (1.7g, 4.5mmol), compound 15 (1.0g, 4.5mmol) in N,N-dimethylformamide (20mL), add N,N,N',N'- Tetramethyl-O-(7-azabenzotriazol-1-yl)urea hexafluorophosphate (2.1 g, 5.4 mmol) and N-methylmorpholine (1.5 mL, 13.5 mmol), maintained at 0°C 3h. Add saturated ammonium chloride solution (20mL) to quench, extract with ethyl acetate (30mL×3), wash with saturated brine (30mL), dry over anhydrous sodium sulfate, filter, concentrate, make sand, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com