Method for extracting lithium from high-sodium lithium-containing brine
A brine and high-sodium technology, applied in the direction of improving process efficiency, can solve the problems of low lithium extraction yield and low separation efficiency, and achieve the effects of increasing lithium concentration, reducing lithium loss, and inhibiting desorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
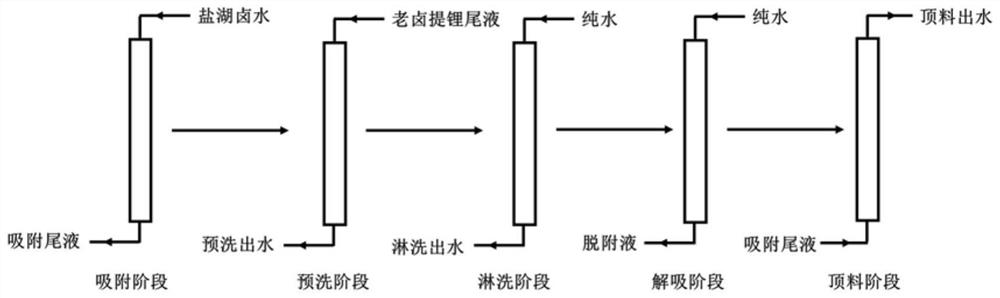
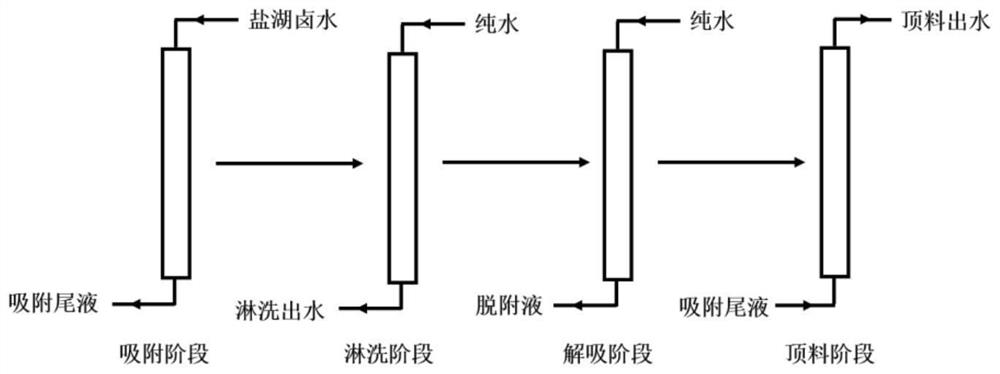
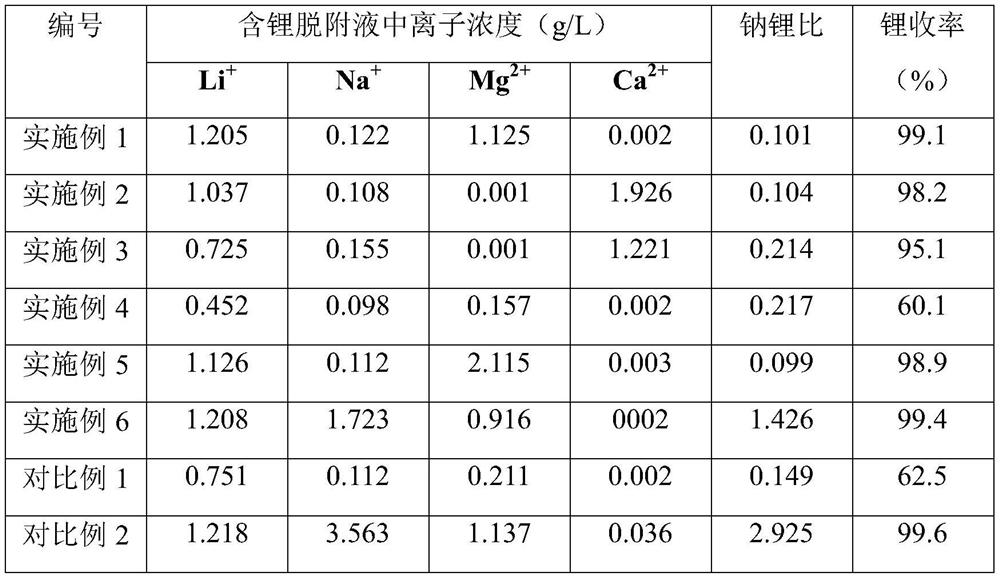
[0033] In this embodiment, a certain salt lake brine is used as a raw material, and the concentrations of lithium ions and sodium ions in the brine are 4.9 g / L and 90.5 g / L, respectively. At 5°C, send the above-mentioned salt lake brine into a resin column equipped with an aluminum-based adsorbent for adsorption at 1BV / h, and collect the adsorption tail liquid; send the tail liquid after lithium extraction from the old brine to the into the column for pre-washing, wherein the concentration of magnesium ions in the tail liquid after extracting lithium from the old halogen is 20.5g / L; use pure water to rinse the pre-washed resin column at 10BV / h; then use pure water to desorb with a flow rate of 30BV / h, collect the lithium-containing desorption liquid; send the adsorption tail liquid from the bottom of the resin column at 0.5BV / h, collect the top material effluent discharged from the top of the resin column, and the top material effluent is used for resin column rinsing or desor...
Embodiment 2
[0036] In this embodiment, a lithium precipitation mother liquor is used as a raw material, and the concentrations of lithium ions and sodium ions in the brine are 1.2 g / L and 76.1 g / L, respectively. Under the condition of 80°C, the above lithium precipitation mother liquor was sent to the resin column equipped with aluminum-based adsorbent for adsorption according to 5BV / h, and the adsorption tail liquid was collected; the calcium chloride solution with a calcium ion concentration of 50g / L was 1BV / h is sent to the column for pre-washing; the pre-washed resin column is rinsed with tap water at 1BV / h; then desorbed with tap water at a flow rate of 2BV / h, and the lithium-containing desorption solution is collected; the adsorption tail liquid is collected at 5BV / h is sent from the bottom of the resin column, and the top material effluent discharged from the top of the resin column is collected, and the top material effluent is used for resin column rinsing or desorption.
[0037...
Embodiment 3
[0039] In this example, brine from an oil field was used as a raw material, and the concentrations of lithium ions and sodium ions in the brine were 0.2 g / L and 118.5 g / L, respectively. At 20°C, send the above-mentioned oilfield brine into a resin column equipped with an aluminum-based adsorbent at a rate of 25BV / h for adsorption, and collect the adsorption tail liquid; send the brine nanofiltration concentrated liquid into the column at a flow rate of 5BV / h Carry out pre-washing, wherein the calcium ion concentration of the brine nanofiltration concentrate is 15.2g / L; rinse the pre-washed resin column with industrial fresh water at 6BV / h; then desorb with industrial fresh water at a flow rate of 15BV / h, and collect the Attached liquid: send the adsorption tail liquid from the bottom of the resin column at 3BV / h, collect the top material effluent discharged from the top of the resin column, and the top material effluent is used for resin column rinsing or desorption.
[0040] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com