Preparation method of 4-benzyloxy phenyl ethyl n-decanoate
A technology of n-decanoate and benzyloxybenzene, applied in the field of preparation of 4-benzyloxyphenylethyl n-decanoate, can solve the problems of many by-products, long reaction route, easy passivation, etc. The effect of improving production efficiency, simplifying production process and avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention is described in further detail below in conjunction with embodiment.
[0022] A kind of preparation method of 4-benzyloxyphenylethyl n-decanoate disclosed by the invention comprises the following steps:
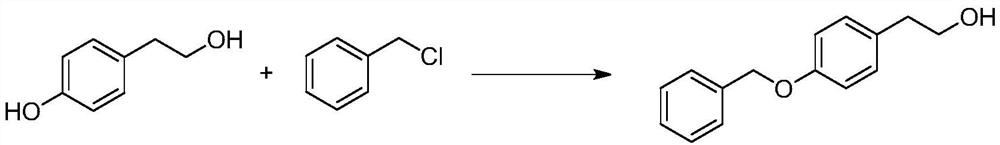
[0023] A. Add methanol, p-hydroxyphenethyl alcohol and equivalent benzyl chloride into a clean three-necked flask, stir and mix evenly, then add solid sodium hydroxide in three batches, heat up to reflux after each addition and react for 0.5 hours, Then cool down to 50 degrees and add the next batch of sodium hydroxide, the reaction equation is as follows:
[0024]
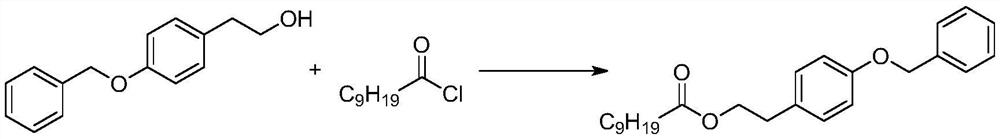
[0025] B. After the step A reaction is completed, the solvent is directly distilled out, and then after the solvent is completely distilled, the equivalent of n-decanoyl chloride is directly added dropwise, and the temperature is controlled below 20 degrees. The reaction equation is as follows:
[0026]
[0027] C. After step B is reacted for 1 hour, a large amount of water is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com