Tetrahydroindazole compound as well as preparation method and application thereof
A technology of tetrahydroindazole and hydrazine compounds, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve problems such as life health and mental health threats, achieve low IC50 value, and strongly inhibit tumor cell growth activity , the effect of inhibiting cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The 4-(4-keto-3,6,6-trimethyl-1-tetrahydroindazole)-2-(4-hydroxycyclohexylamino)benzamide (compound II) prepared above is used to prepare the compound of the present invention Compound I, the method is as follows:
[0041] Take 4-(4-keto-3,6,6-trimethyl-1-tetrahydroindazole)-2-(4-hydroxycyclohexylamino)benzamide (2.0g, 4.87mmol, 1eq) dissolved in In 20mL of ethanol, add hydrazine hydrochloride (0.73g, 10.6mmol, 2.2eq) and sodium acetate (0.80g, 9.7mmol, 2eq) and react at 80°C for 10h. After cooling, a solid precipitates out and is suction filtered to obtain a light yellow solid. Recrystallization from ethyl acetate gave 1.86 g of the product, a 90% yield, melting point 283.5-285.0°C. The product is said compound I, and the synthetic reaction formula is as follows:
[0042]
[0043] 1H NMR (500 MHz, DMSO) δ (ppm) 8.37 (d, 1H), 7.84 (s, 1H), 7.70 (d,1H), 7.15 (s, 1H), 6.62-6.72 (m, 2H), 5.96 (s, 2H), 4.59 (s, 1H), 2.74 (s,2H), 2.38 (s, 3H), 2.29 (s, 2H), 2.01 (d, 2H...
Embodiment 2
[0045] The 4-(4-keto-3,6,6-trimethyl-1-tetrahydroindazole)-2-(4-hydroxycyclohexylamino)benzamide (compound II) prepared above is used to prepare the compound of the present invention Compound I, the method is as follows:
[0046] Take 4-(4-keto-3,6,6-trimethyl-1-tetrahydroindazole)-2-(4-hydroxycyclohexylamino)benzamide (2.0g, 4.87mmol, 1eq) dissolved in In 20mL of ethanol, add 85% hydrazine hydrate (0.46g, 14.6mmol, 3eq) and sodium acetate (1.19g, 14.6mmol, 3eq) and stir and heat at 80°C for 10h. After cooling, a solid precipitates out, which is filtered by suction to obtain light The yellow solid was recrystallized from ethyl acetate to obtain 1.98 g of the product, the yield was 95.5%, and the melting point was 284.5-286.0°C. The HPLC analysis product was consistent with the product of Example 1, and the product was the compound I.
Embodiment 3
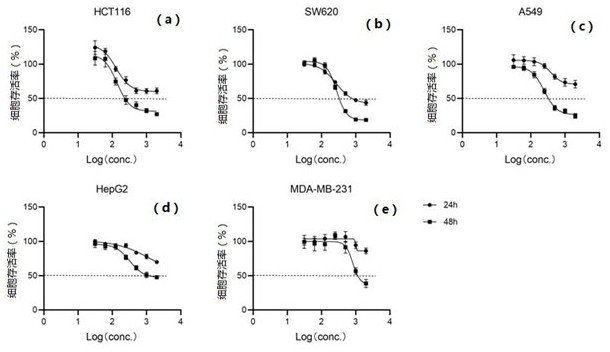
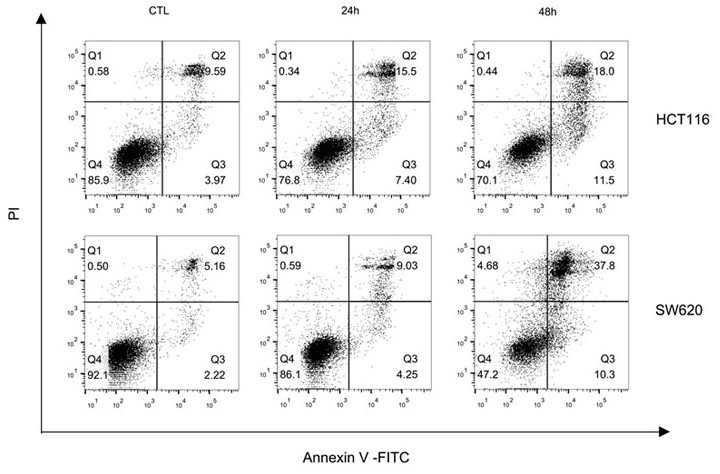
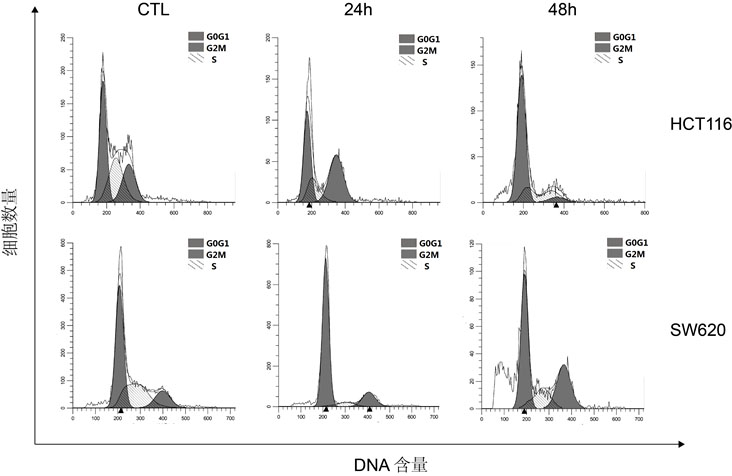
[0048] The growth curve and IC50 value determination of each compound inhibiting tumor cells:
[0049] (1) HepG2, MDA-MB-231, Sw620, A549 and Hct116 were selected as tumor cells to be tested. The culture conditions of HepG2 and MDA-MB-231 cell lines were DMEM medium supplemented with 10% FBS, Sw620 and A549 cell lines The culture condition of the Hct116 cell line was RPMI1640 medium supplemented with 10% FBS, and the culture condition of the Hct116 cell line was McCoy's5A medium supplemented with 10% FBS. All cell lines were stored in 5% CO 2 cultured in a 37°C incubator. Cell viability and growth were assayed in 96-well plates.
[0050] (2) After the cells adhere to the wall, add different concentrations of Compound I, Compound II and GA (Geldanamycin) prepared in Example 1 to each well as the experimental group, and those without adding any drugs as the control Group, cultured for 24h and / or 48h respectively, then added 10μLCCK8 to each well and cultured for 2h, detected ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com