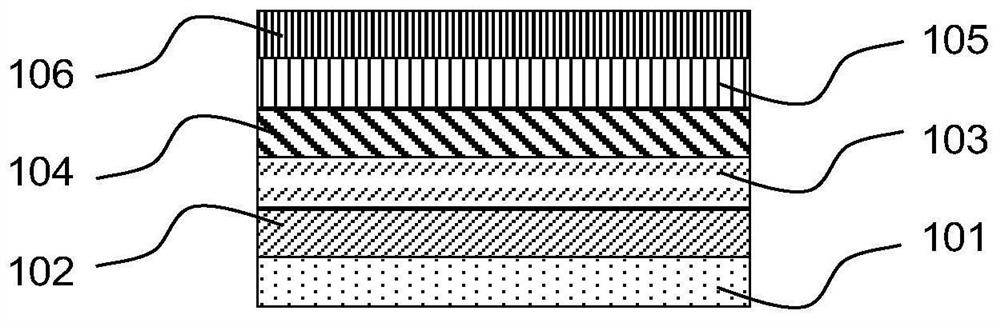
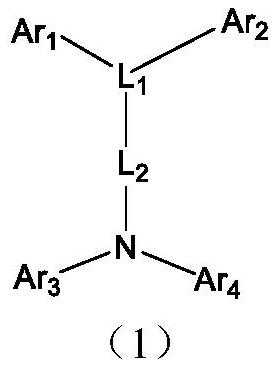
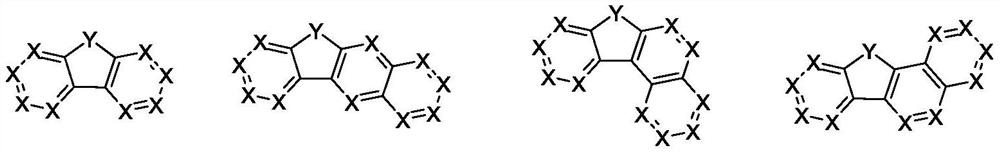Arylamine compound and application thereof in organic electronic device
A compound and aromatic amine technology, applied to aromatic amine compounds and their application in organic electronic devices, can solve the problems of interfacial miscibility, interfacial erosion, etc., and achieve good solubility, good film formation, and long device life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Embodiment 1: the synthesis of compound HT-1
[0181]
[0182] Synthesis of intermediate 3
[0183] Carbazole (119.7mmol), 1,3-dibromo-5-fluorobenzene (143.7mmol), cesium carbonate (239.4mmol), and DMF 200ml were added to a 500ml two-necked bottle successively, and N 2 React overnight at 140 degrees under protection. After the plate reaction, cool down to room temperature, pour the reaction solution into 1000ml of water, precipitate a large amount of solids, filter, wash the filter cake three times, drain it, DCM / PE=1 / 4 beating to obtain 38g of the crude product of compound 3, and feed directly to the next step .
[0184] Synthesis of intermediate 5
[0185] Compound 3 (29.96mmol), compound 4 (29.96mmol), sodium carbonate (59.9mmol), tetrakistriphenylphosphine palladium (1g), toluene 150ml and water 50ml were added in the 500ml two-necked flask successively, N 2 React overnight at 75°C under protection. After the reaction of the boric acid was completed, the pla...
Embodiment 2
[0188] Embodiment 2: the synthesis of compound HT-2
[0189]
[0190] Synthesis of Compound HT-2
[0191] Compound 5 (10mmol), compound 7 (12mmol), sodium carbonate (35.1mmol), tetrakistriphenylphosphine palladium (0.405g), toluene 150ml and water 50ml were added in the 500ml two-necked bottle successively, N 2 React overnight at 95°C under protection. After the reaction of the boric acid was completed, the plate was cooled to room temperature, the liquid was separated, and the water phase was extracted twice with DCM. The organic phases were combined, evaporated to dryness under reduced pressure, mixed with silica gel and passed through the column, the product was passed through DCM / PE=1 / 2, evaporated to dryness under reduced pressure, and then recrystallized from DCM and PE to obtain compound HT-2 with a yield of 76%.
Embodiment 3
[0192] Embodiment 3: the synthesis of compound HT-3
[0193]
[0194] Synthesis of Intermediate 9
[0195] Compound 3 (30mmol), compound 8 (30mmol), sodium carbonate (60mmol), tetrakistriphenylphosphine palladium (1g), toluene 150ml and water 50ml are added in the 500ml two-necked flask successively, N 2 React overnight at 75°C under protection. After the reaction of the boric acid was completed, the plate was cooled to room temperature, the liquid was separated, and the water phase was extracted twice with DCM. The organic phases were combined, evaporated to dryness under reduced pressure, mixed with silica gel and passed through the column, and the product was passed through with DCM / PE=1 / 3, and evaporated to dryness under reduced pressure to obtain 6.0 g of compound 9.
[0196] Synthesis of compound HT-3
[0197] Compound 9 (10mmol), compound 10 (12mmol), sodium carbonate (35mmol), tetrakistriphenylphosphine palladium (0.405g), toluene 150ml and water 50ml were added ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com