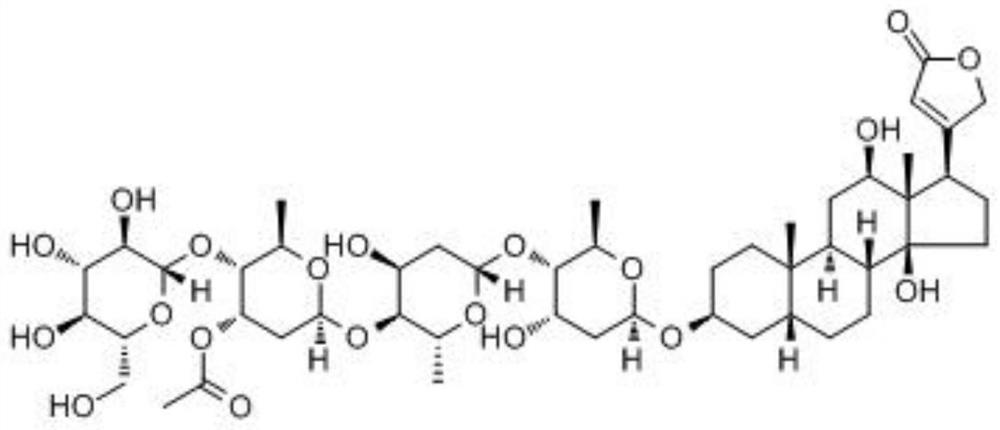
Application of loranoside C as Beclin1 activator in preparation of anti-hepatoma drugs
A technology of lanatoside C and an activator, which is applied in the field of biomedicine and can solve unclear problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
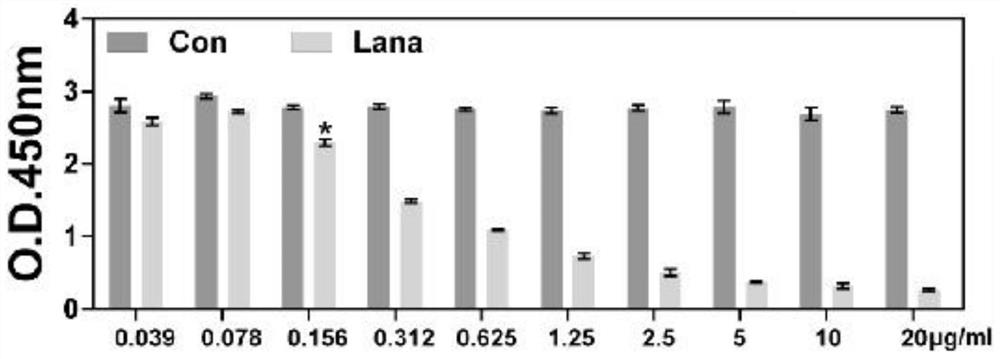
[0029] Embodiment 1, drug IC50 experiment
[0030] (1) After routinely digesting the cells with trypsin at room temperature, blow them into a uniformly distributed single-cell suspension with DMEM medium containing FBS, inoculate them in a 96-well plate at a density of 4,000 cells per well, and continue culturing at 37°C, 5%CO 2 in the incubator;
[0031] (2) After 24 hours of adherent cell growth, lanatoside C was added to make the final concentration 0.039, 0.078, 0.156, 0.312, 0.625, 1.25, 2.5, 5, 10, 20 μg / mL, and the control group was given an equal volume of DMSO ( That is, the DMSO dosage of 20 μg / mL lanatoside C was dissolved), and the cells were cultured for 1 day;
[0032] (3) Discard the cell culture medium, add 10 μL of CCK-8 solution to each well, and continue culturing for 2 h;
[0033] (4) Use a microplate reader to measure the absorbance at 450nm, compare the effects of different doses on cell proliferation inhibition, and calculate the cell proliferation in...
Embodiment 2
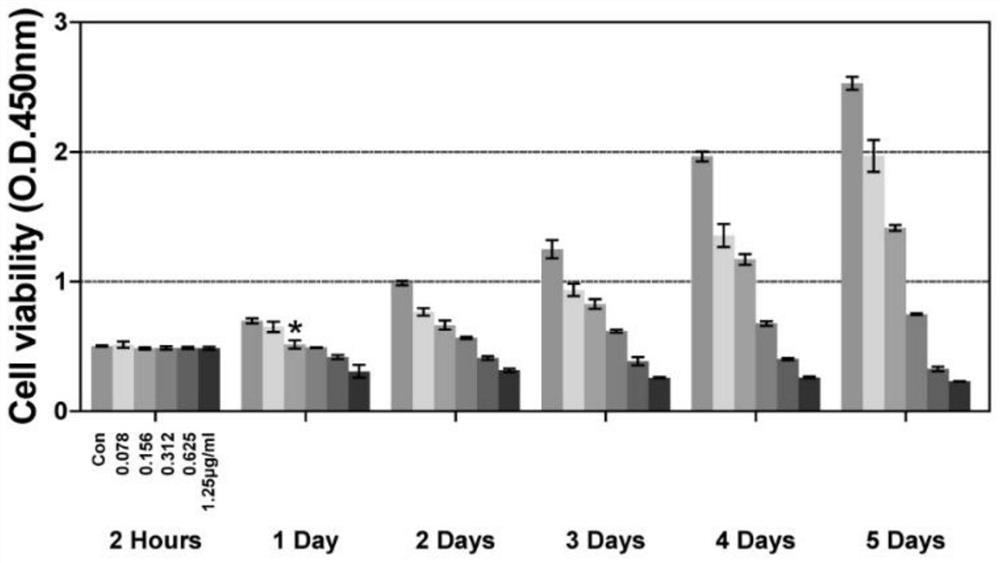
[0035] Example 2. Cell proliferation assay (CCK-8) detects the inhibitory effect of different concentrations of lanatoside C on the proliferation of liver cancer Huh-7 cells at different times
[0036] (1) After routinely digesting the cells with trypsin at room temperature, blow them into a uniformly distributed single-cell suspension with DMEM medium containing FBS, inoculate them in a 96-well plate at a density of 2,000 cells per well, and continue culturing at 37°C. 5%CO 2 in the incubator;
[0037] (2) After 24 hours of adherent cell growth, lanatoside C was added to make the final concentrations 0.078, 0.156, 0.312, 0.625, and 1.25 μg / mL, and the control group was given an equal volume of DMSO (that is, 1.25 μg / mL lanatoside C was dissolved with DMSO dosage of Glycoside C), culture cells for 2h, 1d, 2d, 3d, 4d, 5d;
[0038] (3) Discard the cell culture medium, add 10uL of CCK-8 solution to each well, and continue culturing for 2h;
[0039] (4) Use a microplate reader ...
Embodiment 3
[0041] Example 3. Cell clone formation experiment to detect the effect of lanatoside C on the clone formation of liver cancer Huh-7 cells
[0042] (1) After routinely digesting the cells with trypsin at room temperature, blow them into a uniformly distributed single-cell suspension with DMEM medium containing FBS, inoculate them in a 6-well plate at a density of 2,000 cells per well, and continue culturing at 37°C. 5%CO 2 in the incubator;
[0043](2) After culturing for 9 days, lanatoside C was added to intervene, so that the final concentration was 0.625 μg / mL, and the control group was given an equal volume of DMSO (that is, the cells were treated with DMSO dose of 0.625 μg / mL lanatoside C) , continue to cultivate for 7 days;
[0044] (3) Discard the culture medium in the upper layer, and wash the plate twice with PBS solution;
[0045] (4) Fix the cells with 4% paraformaldehyde for 15 minutes, discard the paraformaldehyde solution;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com