Method for establishing human blood-brain barrier model in vitro through 3D co-culture of four cells
A cell model and blood-brain barrier technology, applied in the field of cell models, can solve the problems of malnutrition, difficult treatment and intervention, growth restriction, etc., and achieve blood-brain barrier function breakthrough, easy treatment and intervention, and cell contact uniform effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Cell Culture and Treatment
[0032] Human primary brain microvascular endothelial cells (hpBEC) were purchased from Angio-Proteomie, USA. Human primary cerebral perivascular cells (hpPs) and primary human cerebral astrocytes (hpA) were purchased from ScienCell, USA.
[0033]Human neuroblastoma cell line SH-SY5Y (American Type Culture Collection) was grown in modified Eagle medium + F12 cell medium (1:1) (PAA Laboratories, Pasching, Austria), supplemented with 15% fetal Bovine serum, 1% penicillin / streptomycin. HpBECs were grown in endothelial basal medium (EGM-2) (Lonza, UK) supplemented with growth factors and 0.4% fetal calf serum. HpPs were grown in Pericyte Growth Supplemented Medium (ScienCell, USA), whereas hpA were grown in Astrocyte Growth Supplemented Medium (ScienCell, USA). HpBECs, hpPs and hpA were used for experiments between passage 2 to passage 4. Co-cultivation was performed by the RAFT 3D cell culture system (Lonza, London, UK) and cells w...
Embodiment 2
[0036] Example 2 Using 4 different cell types to co-culture with or without 6-OHDA and (or) glucose to establish NVU, PD, DM, PD-DM and down-regulated BBB cell models of the PDGFRβ gene
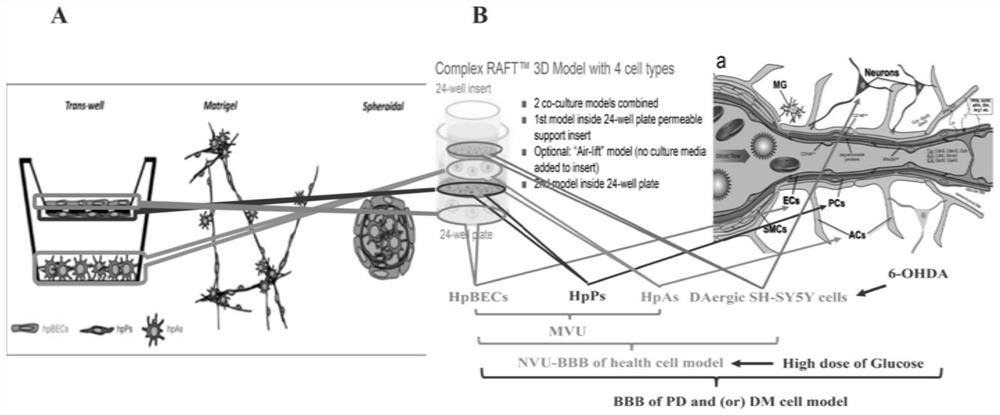
[0037] The present invention first established in vitro the 3D cell culture system (UK, Lonza) of the neurovascular unit (NVU) model composed of hpBEC, hpP, hpA and dopaminergic (DAergic) neuron cells (SH-5Y) (co-cultivation 6 sky). The model of the present invention can well simulate the arrangement of blood-brain barrier cells in vivo according to the order of hpBEC, hpP, hpAs and SH-SY5Y cells from inside to outside ( figure 1 ).
[0038] SH-SY5Y cells were divided into 1.0×10 5 Cells / ml were seeded in 24-well plates for 24 hours. SH-SY5Y cells received different concentrations of 6-OHDA (0, 50, 100, 150μmol / L) at different time points (0,8,24,48h). The concentration of 6-OHDA was selected as 50 μmol / L, and co-cultured for 24 hours (the survival rate of SH-SY5Y cells detected by trypan...
Embodiment 3
[0041] Example 3 Fluorescein Sodium (Na-FLU) Permeability Measurement
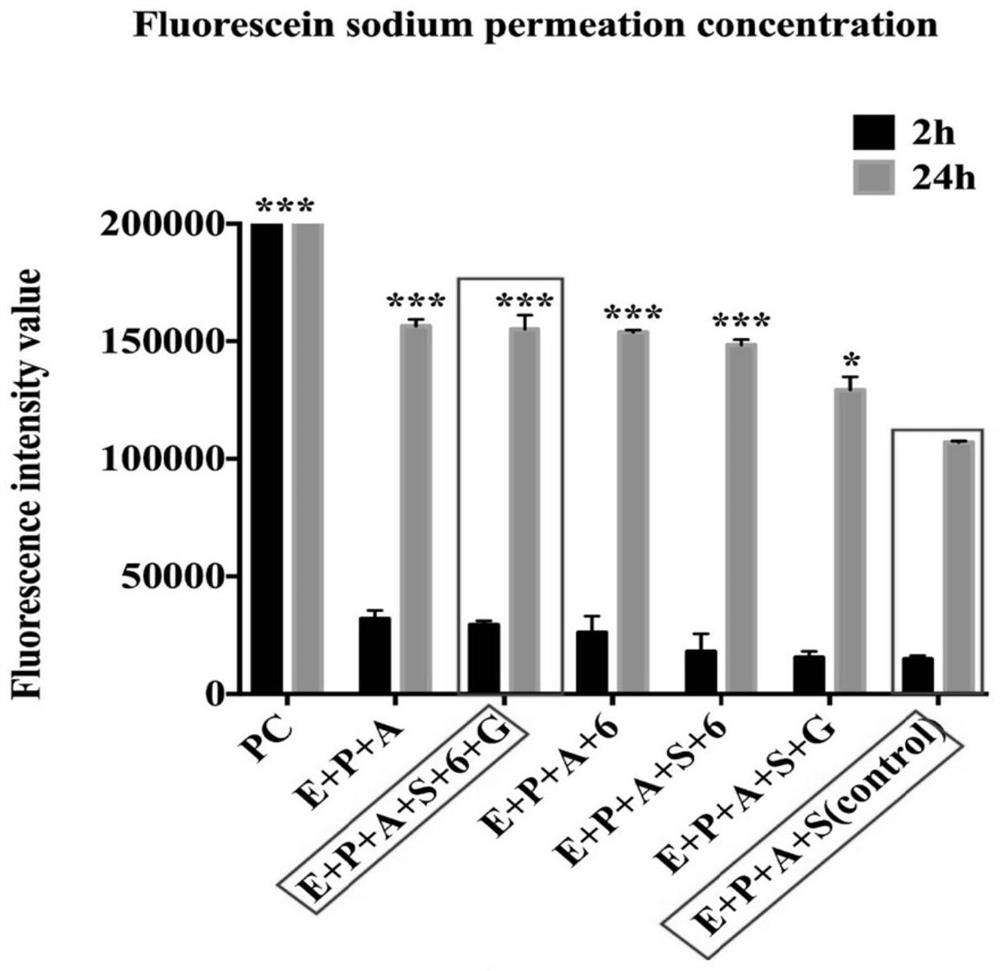
[0042] After the in vitro BBB cell model is completed, wash the inside and outside of the model 2 to 3 times with serum-free EBM-2 medium, and then add 200 μL of Na-FLU at a concentration of 100 mg / L to the inside of each well. Add 1.2 mL of EBM-2 medium externally. After 2 h and 24 h, remove 100 μL of medium from outside the pool. Use a microplate reader to measure the amount of Na-FLU in the BBB model under the condition that the excitation wavelength is 460nm and the emission wavelength is 515nm ( figure 2 ).
[0043] The beneficial effects of this embodiment are: the blood-brain barrier in vivo can be well simulated, and the arrangement order of the cells is consistent with the arrangement order of the blood-brain barrier in vivo, so as to better study the damage of Parkinson's and diabetes on the blood-brain barrier and how to protect it in the future Human blood-brain barrier research. The healt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com