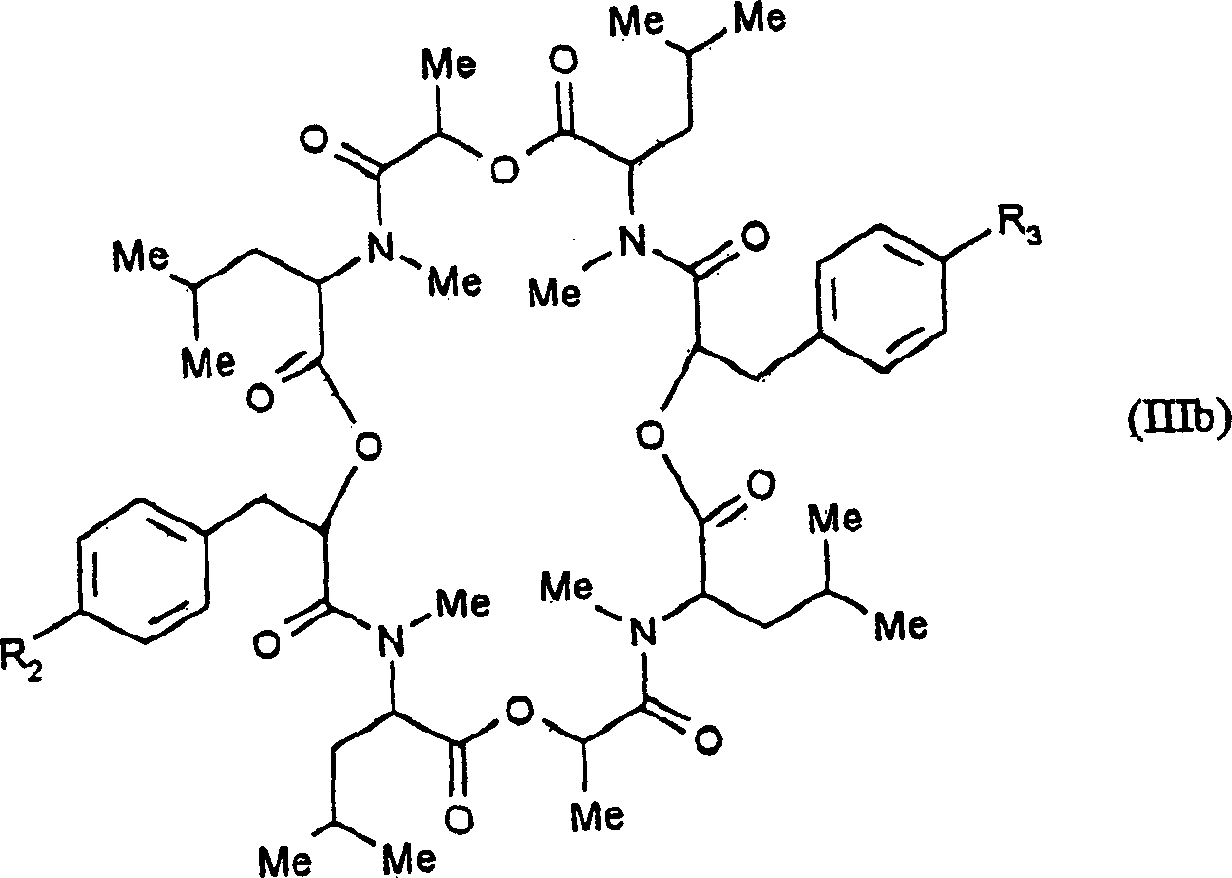
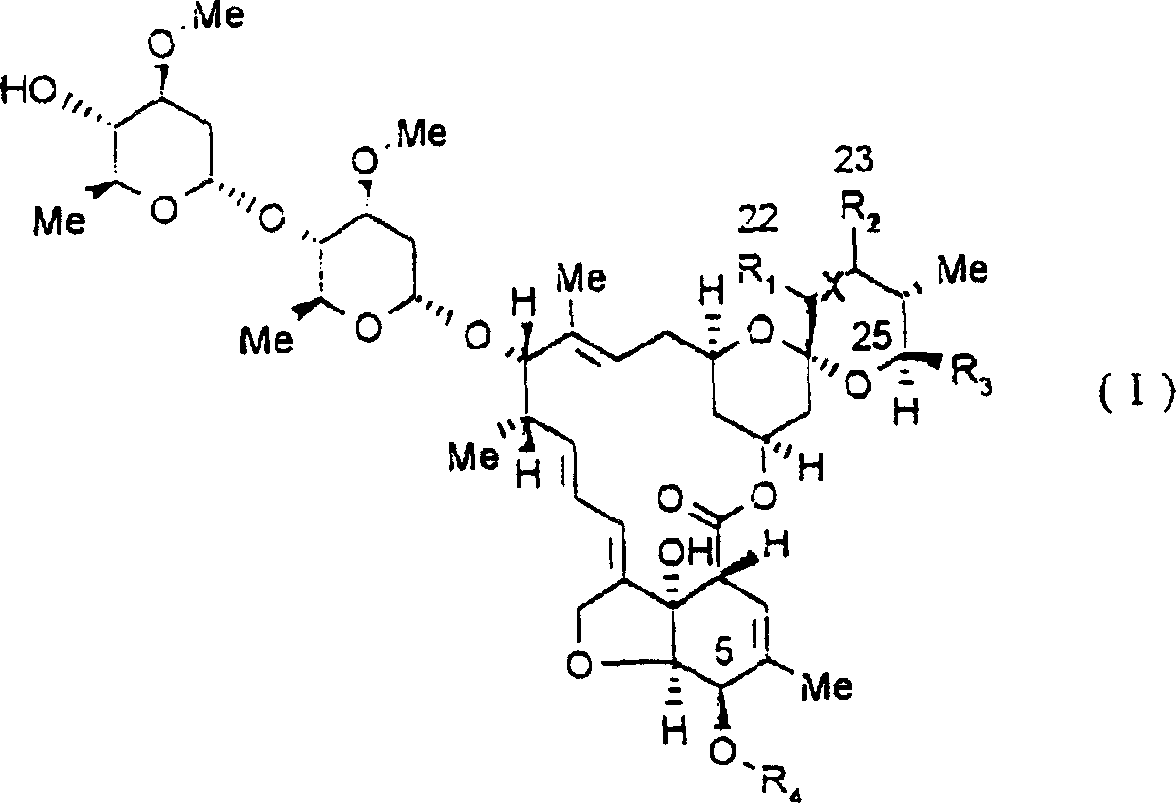
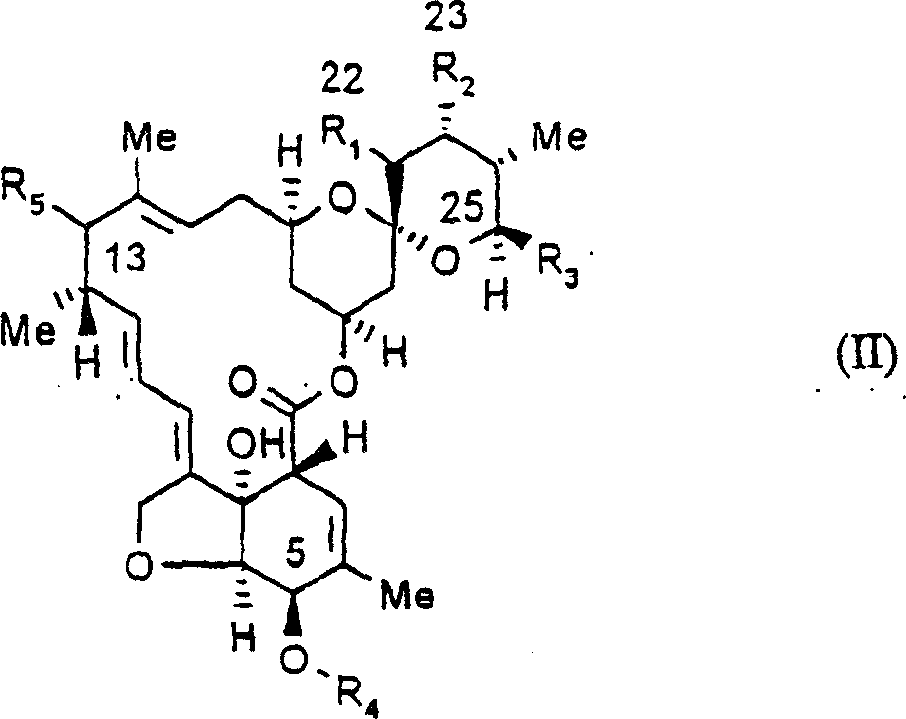
Endoparasiticial composition
A technology for endoparasites and compositions, applied in the field of endoparasite-killing compositions, can solve problems such as inability to control roundworms, hookworms, whipworms and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment A
[0170] Nematode Nematospiroides dubius in mice
[0171] Mice were experimentally infected with the nematode Nemotospiroides dubius. The method of infection was oral administration of 60 filamentous larvae of Nematospiroides dubius.
[0172] After the incubation period, the active substance in suspension was administered orally on the 12th day after infection.
[0173] Activity assay:
[0174] Mice were selected on day 20 post-infection. Count parasites in mouse duodenum using a press. The dose-treated group was successful relative to the no-dose control group.
[0175] Tables A and B below show the effect of the conjugates on the nematode Nematospiroides dubius in mice.
[0176] Active substance and dosage [mg / kg]
[0177] Active substance and dosage
Embodiment B
[0179] In vivo nematode test
[0180] Nematode Heterakis spumosa in mice
[0181] Mice were experimentally infected with the Heterakis spumosa nematode. The method is to take 90 capsules of fertilized eggs of Heterakis spumosa orally.
[0182] After the incubation period, the active substance in suspension was administered orally on day 46 after infection.
[0183] Activity assay:
[0184] Mice were selected on day 59 post-infection. Microscopic enumeration of parasites in mouse colon and cecum. The dose-treated group was successful relative to the no-dose control group.
[0185]Tables C and D below show the effect of the conjugates on the Heterakis spumosa in mice.
[0186] Active substance and dosage [mg / kg]
[0187] Active substance and dosage [mg / kg]
Embodiment C
[0189] In vivo nematode test
[0190] Ancylostoma caninum in dogs
[0191] Small game puppies were experimentally infested with Ancylostoma caninum. The dosage is 250 L 3 larva.
[0192] After the incubation period, when the adult worms had reached the gastrointestinal tract of the dogs, the dogs were given orally a capsule of the pure active compound tested.
[0193] Drug efficacy determination is carried out according to the following two methods:
[0194] 1. Enumeration of eggs in dog feces before and after treatment
[0195] 2. Use the following formula to calculate the percentage efficiency of the critical test
[0196]
[0197] Table E shows the effect of the conjugates against the hookworm Ancylostoma caninum in dogs.
[0198] Active substance and dosage [mg / kg]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com