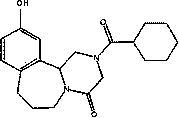
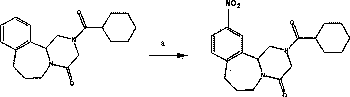Preparation of 10-hydroxy epsiprantel and applications thereof
A technology of hydroxyiqualone and iqualone, applied in the field of drug discovery, can solve problems such as resistance, drug resistance, and sensitivity decline
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019]
[0020]
[0021] See existing literature reports (Bioorg.Med.Chem.Lett., 2007, 17, 4154-4157), the preparation of compounds with amino groups specifically includes:
[0022] Step a: Synthesis of Compound 1
[0023] Iquinone (3.00 g, 9.60 mmol) was dissolved in sulfuric acid (9.42 g, 5.14 mL, 96 mmol, 10 eq) cooled to 0°C. Concentrated nitric acid (3.03 g, 2.12 mL, 48 mmol, 5 eq) was then carefully added. The reaction mixture was stirred at room temperature for 24 hours, cooled with ice, and neutralized with sodium carbonate to pH 8. The reaction mixture was extracted 3 times with EtOAc (150 mL). The combined organic phases were dried with MgSO4, the organic solvent was removed under reduced pressure, and the product was purified with silica gel column (petroleum ether: ethyl acetate 5:1). The product was obtained as a yellow solid 1.34 g, with a yield of 39%. Compound 1 is analyzed, and the data are as follows: R F (EtOAc:Pet, 3:1) 0.23; m.p. 88...
Embodiment 2 Embodiment 1
[0027] Example 2 Insecticidal activity test of the gained 10-hydroxyl quinone in vitro of embodiment one
[0028] The portal vein infusion method was used to infect mice with Schistosoma japonicum C. japonicum, and the juvenile and adult worms were collected at the juvenile stage (16-18 days after infection) and adult stage (6 weeks after infection), and the synthesized compound 10- Hydroxyiqualone against schistosomiasis juvenile and adult worms.
[0029] (1) Observing the killing effect of the iqualone derivative 10-hydroxyiqualone on schistosome larvae: the compound 10-hydroxyiqualone at a concentration of 1-50 μM was used to kill schistosome larvae. At the same time, as a reference, set up a blank control group, an iqualone group, and an artesunate group, and then observe the death of schistosomiasis larvae under the light microscope. The action of 10-hydroxyiqualone caused spastic contraction and then died; the worms in the blank control group and the iqualone cont...
Embodiment 3 Embodiment 1
[0030] Example 3 Anti-schistosomiasis adult worm effect test in vivo of the compound 10-hydroxyl quinone obtained in embodiment 1
[0031] Three groups were set up for the experiment, one group was the iquinone positive control group, one group was the compound 10-hydroxy iquinone group, and the third group was the negative control group (no administration), with 10 mice in each group. Each mouse was infected with 60 tail butterflies, and on the 28th day after the infection, the mice were orally administered 10-hydroxyiquinone (amount of 200 mg / kg, that is, 200 mg per 1 kg according to the weight of the mice), for 5 consecutive days. Day; the mice in the iqualone control group were orally administered iqualone 100 mg / kg for 5 consecutive days; the mice in the three groups were dissected on the 21st day after the administration, the hepatic portal vein was perfused aseptically, and the worms were counted. As a result, it was found that the schistosomes in the normal contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com