Silver-doped copper vanadate composite photocatalytic material, preparation method thereof and application of silver-doped copper vanadate composite photocatalytic material as carbon dioxide reduction photocatalyst
A technology of composite photocatalysis and copper vanadate, which is applied in the direction of carbon monoxide, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc. Catalytic activity is not very high, photocatalysis is rarely reported, etc., to achieve high catalytic activity and stability, increase adsorption capacity, and broaden the photoresponse range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 (comparative example)
[0046] CuV 2 o 6 Preparation of nanostructures: Weigh 0.402g of Cu(NO 3 ) 2 ·3H 2 Dissolve the O solid in 30ml of distilled water, stir and dissolve at room temperature until the yellow clear solution is recorded as A solution; weigh 0.303g of V 2 o 5 Disperse the solid in 30ml of pure water, stir at room temperature until the dispersion is uniform, and record it as B suspension; add B suspension to A solution drop by drop, continue stirring for 30 minutes, and record it as C suspension; adjust the pH at the same time =6, transfer the C suspension to a 100ml reactor, place the reactor in an oven, and react at a high temperature of 220°C for 24h. The yellow product obtained after the reaction was washed with water and ethanol, and then transferred to a vacuum drying oven at 50° C. for 6 h.
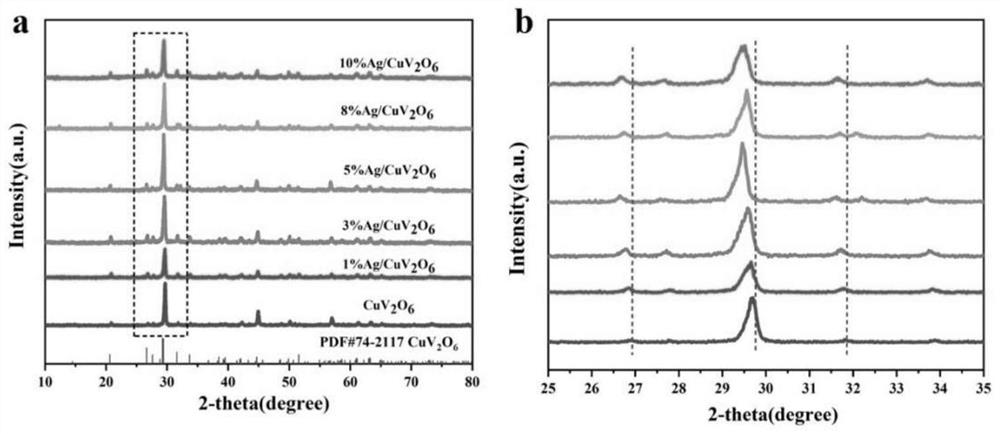
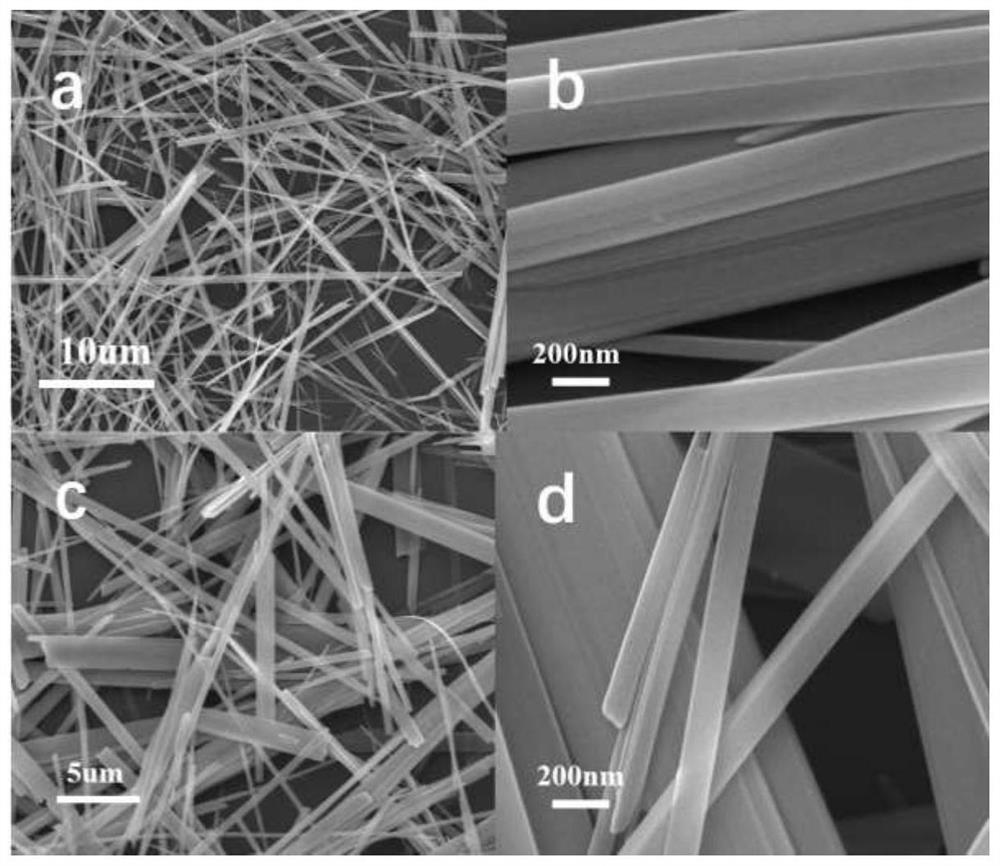
[0047] For the CuV prepared in embodiment 1 2 o 6 Some crystal structure and morphology studies were performed. Depend on figure 1 It ...
Embodiment 2
[0049] Silver doped CuV 2 o 6 Preparation of composite photocatalyst: weigh 0.402g of Cu(NO 3 ) 2 ·3H 2 Dissolve the O solid in 30ml of distilled water, stir and dissolve at room temperature until the yellow clear solution is recorded as A solution; weigh 0.303g of V 2 o 5 Disperse the solid in 30ml of pure water, stir at room temperature until the dispersion is uniform, and record it as B suspension; add B suspension to A solution drop by drop, continue stirring for 30 minutes, and record it as C suspension; adjust the pH at the same time =6. After stirring for 30 minutes, add different amounts of 0.1M silver nitrate solution, which is recorded as D solution, and continue stirring for 30 minutes. The C solution was transferred to a 100ml reaction kettle, and the reaction kettle was placed in an oven, and reacted at a high temperature of 220° C. for 24 hours. The yellow product obtained after the reaction was washed with water and ethanol, and then transferred to a vacu...
Embodiment 3
[0050] Embodiment 3 (comparative example)
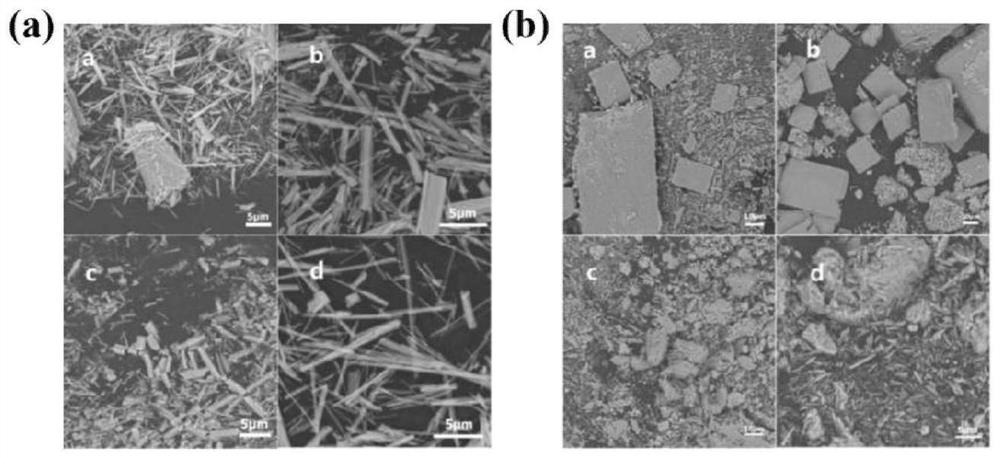
[0051] CuV 2 o 6Nanostructures were prepared under different temperature and time conditions: Weighed 0.402g of Cu(NO 3 ) 2 ·3H 2 Dissolve the O solid in 30ml of distilled water, stir and dissolve at room temperature until the yellow clear solution is recorded as A solution; weigh 0.303g of V 2 o 5 Disperse the solid in 30ml of pure water, stir at room temperature until the dispersion is uniform, and record it as B suspension; add B suspension to A solution drop by drop, continue stirring for 30 minutes, and record it as C suspension; adjust the pH at the same time =6, transfer the C suspension to a 100ml reaction kettle, place the reaction kettle in an oven, and react at a high temperature of 80-180°C for 4-16 hours. The yellow product obtained after the reaction was washed with water and ethanol, and then transferred to a vacuum drying oven at 50° C. for 6 h. The morphology of the sample prepared in Example 3 was explored to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com