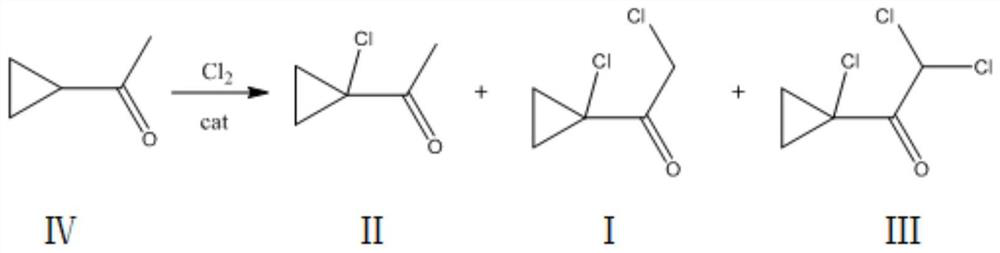
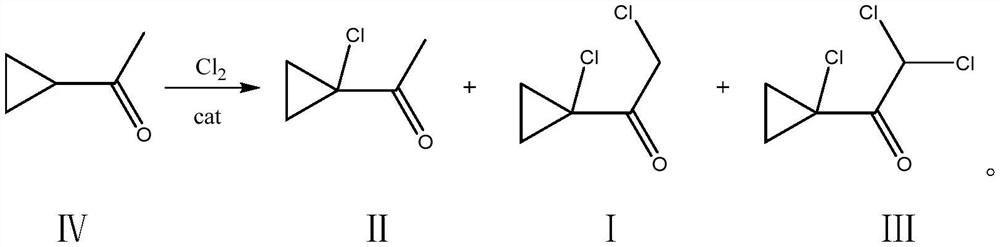
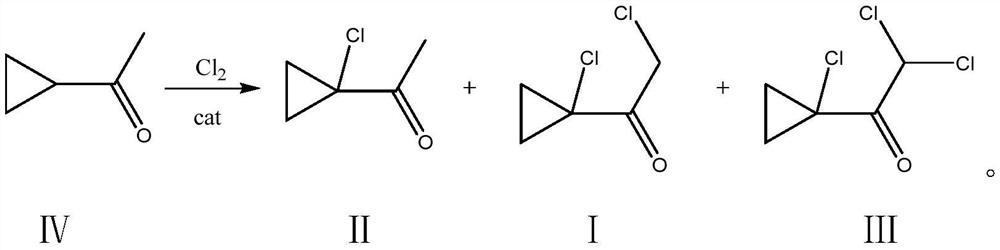
Synthesis method of 2-chloro-1-(1-chlorocyclopropyl) ethanone
A technology of chlorocyclopropyl group and a synthesis method, applied in the synthesis field of 2-chloro-1-ethanone, can solve the problems of poor selectivity, low yield, difficult control of impurities, etc., and achieves improved yield and less corrosiveness of equipment , improve the effect of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 100g of dichloroethane and (200g, 2.378mol) cyclopropylmethyl ketone into a 500mL four-neck flask, cool down to -5°C, and add (1.34g, 11.8mmol) MeAlCl under stirring 2 , Feed chlorine gas, feed rate is about 25g / h, tail gas water carries out three-stage absorption, ventilates 14.0h, gas chromatography detects product relative content 2-chloro-1-(1-chlorocyclopropyl) ethyl ketone 4.2%, 2-Chloro-1-(1-chlorocyclopropyl)ethanone 93.8%, 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone 1.8%, slowly heated to 30°C under reduced pressure Remove solvent. Under -0.1MPa vacuum, use a 50cm rectification column and a glass ring packing to carry out rectification reaction, slowly raise the temperature to 130°C, collect the fraction at 100-102°C to obtain 2-chloro-1-(1-chlorocyclopropyl) Ethanone, 82.5~82.6°C, the previous fraction was applied back to the reaction to obtain 341.7 g of the product 2-chloro-1-(1-chlorocyclopropyl)ethanone, with a content of 97% and a yield of 91.0%.
...
Embodiment 2
[0031] Add (200g, 2.378mol) cyclopropylmethyl ketone into a 500ml four-necked flask, cool down to 10°C, add 150g dichloroethane, and add (4.4g, 47.6mmol) Me 2 AlCl, feed chlorine gas, the feed rate is about 60g / h, tail gas is absorbed by water for tertiary absorption, reacted for 6.5h, and the relative content of the product detected by gas chromatography is 5.4% of 2-chloro-1-(1-chlorocyclopropyl)ethanone , 2-chloro-1-(1-chlorocyclopropyl)ethanone 93.5%, 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone 0.9%, slowly warming up to 30°C under reduced pressure Solvent was removed. Under -0.1MPa vacuum, use a 50cm rectification column and a glass ring packing to carry out rectification, slowly raise the temperature to 130°C, collect fractions at 99.6~102°C, and obtain 2-chloro-1-(1-chlorocyclopropyl) Ethyl ketone, 82.5-82.7 ° C front fraction was applied back to the reaction to obtain 339.6 g of the product, the content was 96%, and the yield was 89.6%.
Embodiment 3
[0033] Add (200g, 2.378mol) cyclopropylmethyl ketone into a 1000ml four-necked flask, cool down to 15°C, add 200g of dichloromethane, and add (8.95g, 95mmol) MeAlCl under stirring condition 2 and (3.67g, 39.6mmol) Me 2 AlCl, feed chlorine gas, feed rate is about 75g / h, tail gas water carries out tertiary absorption, reacts 3.5h, gas chromatography detects product 2-chloro-1-(1-chlorocyclopropyl) ethyl ketone 2.4%, 2 -Chloro-1-(1-chlorocyclopropyl)ethanone 95.2%, 2,2-dichloro-1-(1-chlorocyclopropyl)ethanone 2.1%, the reaction is completed and the temperature is slowly raised to 30°C under reduced pressure Remove dichloromethane, under -0.1MPa vacuum, use 50cm rectification column, glass ring packing to carry out rectification, slowly heat up to 130°C, collect fractions at 100-102°C to obtain 2-chloro-1-(1 -Chlorocyclopropyl)ethanone, 82.4~82.8°C front fraction was applied back to the reaction to obtain 343.4g of product, the content was 96%, and the yield was 90.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com