Acarbose solid oral preparation and preparation method thereof
A technology for acarbose and oral preparations, which is applied in the field of acarbose solid oral preparations and its preparation, can solve problems such as quality differences between batches, and achieve the effects of low cost, extended validity period, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0056] According to the ICH guidelines and the Chinese Pharmacopoeia 2020 edition four drug stability research guidelines, the influence factor test and forced degradation test are the main means to study the degradation pathway of drug degradation impurities, combined with the factors that may be involved in the acarbose preparation process and storage process Analysis shows that temperature and moisture are the main reasons for the stability of the product. Therefore, raw materials provided by two different suppliers are taken. Among them, the representative of the domestic raw material supplier: Hebei Huarong, batch number: 04160903; the imported raw material: Korea CKD Bio , Batch number: BAE003A, after adding water to dissolve (concentration: 20mg / ml) high temperature 90 ℃ water bath for 8 hours, check the color change of the solution, and detect related substances. One of the two suppliers of raw materials is an imported raw material drug, and the other is a supplier of a...
experiment example 2
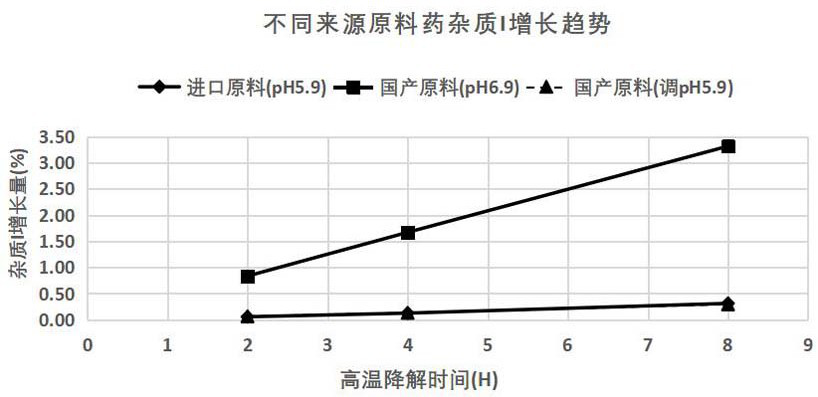
[0063] According to the above results, comparing the test results and stability of raw materials from different suppliers, and the pH measurement results of the same concentration of acarbose solution, the pH of the solution of domestic acarbose raw material is adjusted to be the same as that of imported acarbose solution. Same pH, re-do the high temperature hydrolysis damage test (results are shown in Table 3 and Figure 5 shown). Sampling and detection of related substances in 2 hours, 4 hours, and 8 hours of high-temperature destruction respectively, and drawing the growth trend curve of impurity I of raw material medicine from different sources during high-temperature hydrolysis (see figure 1 ).
[0064]
[0065] According to the above results, when the pH of the aqueous solution of domestic raw materials (20 mg / ml) is adjusted to the pH of the imported raw materials, it is hydrolyzed at a high temperature of 90 °C for 8 hours, and the degradation range of impurities I...
experiment example 3
[0070] In view of the fact that the pH of acarbose raw materials in the quality standards of acarbose raw materials recorded in the official pharmacopoeias of various countries is pH5.5~pH7.5, the following experiments are designed to further confirm the stable pH range of raw materials. The specific steps are as follows:
[0071]
[0072] The above prepared solutions were respectively placed in a 90°C water bath and heated for 8 hours, and the detection method in Experimental Example 1 was used to detect related substances. The results are shown in Table 6 below:
[0073]
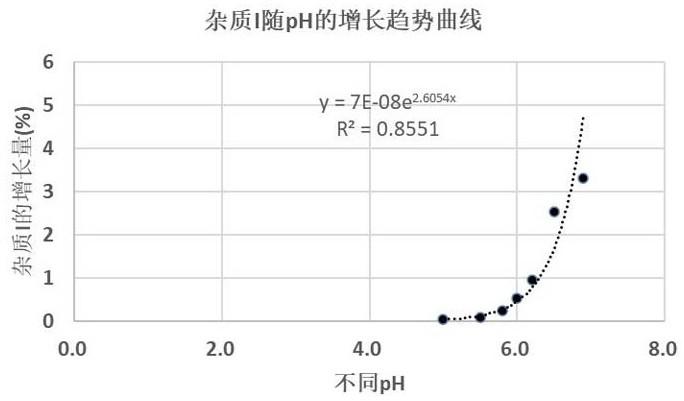
[0074] According to above-mentioned table 6 result, draw impurity I along with the growth trend curve of pH, result is as follows figure 2 shown.
[0075] According to the above results, as the pH of the solution gradually decreased from pH6.9 to pH5.0, after 8 hours of high-temperature degradation at 90°C, the detected amount of impurity I decreased from 3.47% to 0.19%, and the detected amount of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com