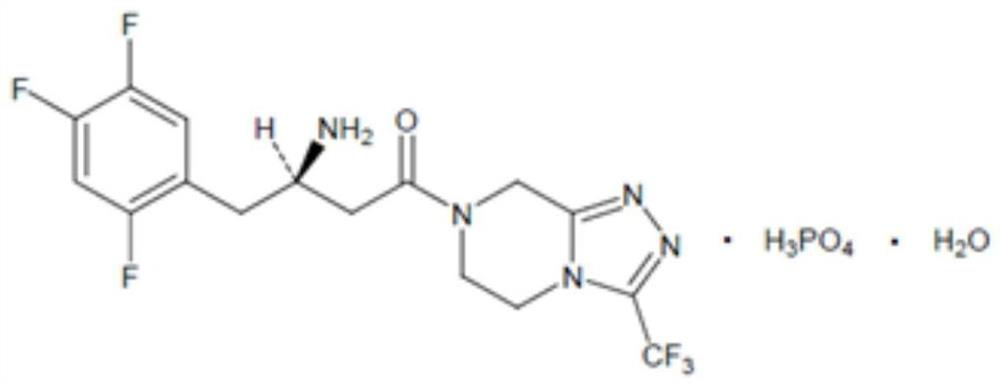
Sitagliptin metformin tablet preparation and preparation method thereof
A technology of metformin and metformin hydrochloride, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients. Production cost and process complexity, increased secretion of insulin and glucagon, etc., to achieve the effects of good fluidity, excellent content uniformity, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
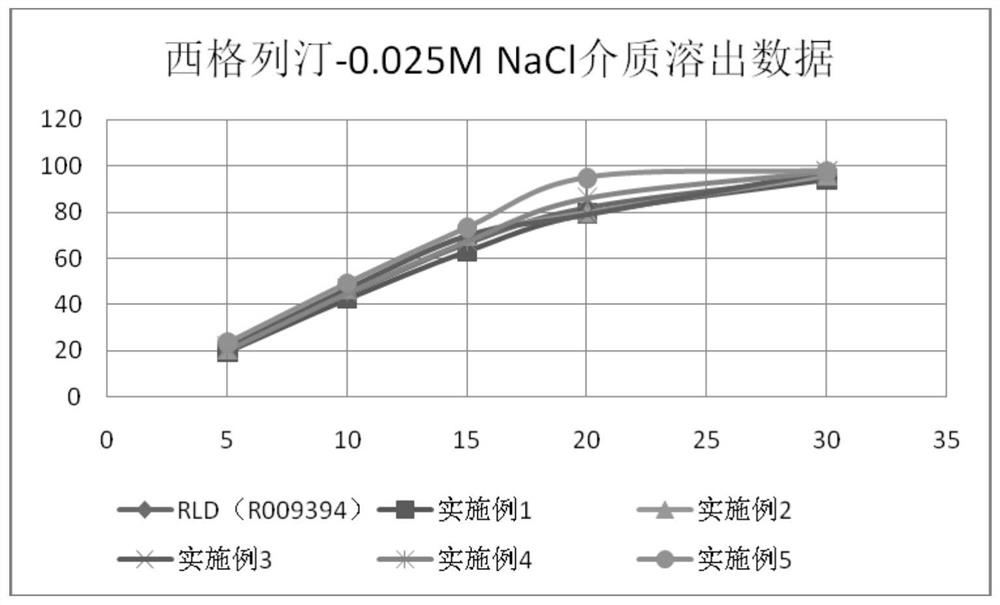
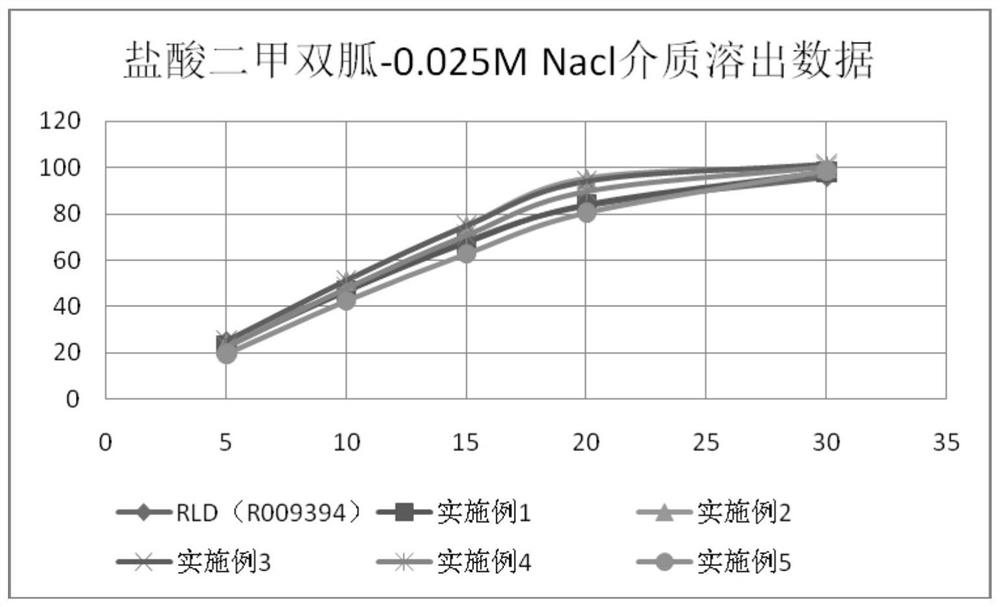
Image
Examples
Embodiment 1
[0032] Embodiment 1: preparation of sitagliptin metformin tablet preparation of the present invention
[0033] 1. Prescription
[0034] Specifications per tablet: 50mg / 850mg, prescription quantity 3000 tablets, see Table 1 for details;
[0035] Table 1
[0036]
[0037] Note: Opadry powder is mixed with pure water to prepare 15% coating liquid before use for coating.
[0038] 2. Preparation process
[0039] Step 1: take metformin hydrochloride and pulverize through a 100-mesh sieve;
[0040] Step 2: mixing metformin hydrochloride with the binder to obtain mixture 1;
[0041]Step 3: Set the hot-melt extrusion temperature to 120°C-140°C, and the extrusion speed to 300rpm-500rpm. After the temperature reaches the set temperature, take the mixture 1 for hot-melt extrusion to obtain an extrudate.
[0042] Step 4: cooling the extrudate obtained in step 3, cutting, sieving and pulverizing;
[0043] Step 5: Mix the granules obtained in Step 4 with sitagliptin phosphate monohy...
Embodiment 2
[0044] Embodiment 2: preparation sitagliptin metformin tablet preparation
[0045] 1. Prescription
[0046] Specifications per tablet: 50mg / 850mg, prescription quantity 3000 tablets, see Table 2 for details;
[0047] Table 2
[0048]
[0049]
[0050] Note: Opadry powder is mixed with pure water to prepare 15% coating liquid before use for coating.
[0051] 2. Preparation process
[0052] With embodiment 1.
Embodiment 3
[0053] Embodiment 3: preparation sitagliptin metformin tablet preparation
[0054] 1. Prescription
[0055] Specifications per tablet: 50mg / 850mg, prescription quantity 3000 tablets, see Table 3 for details;
[0056] table 3
[0057]
[0058] Note: Opadry powder is mixed with pure water to prepare 15% coating liquid before use for coating.
[0059] 2. Preparation process
[0060] With embodiment 1.
[0061] Embodiment: 4: prepare sitagliptin metformin tablet preparation
[0062] 1. Prescription
[0063] The specification of each tablet is 50mg / 850mg, and the prescription quantity is 3000 tablets, see Table 4 for details;
[0064] Table 4
[0065]
[0066]
[0067] Note: Opadry powder is mixed with pure water to prepare 15% coating liquid before use for coating.
[0068] 2. Preparation process
[0069] Step 1: take metformin hydrochloride and pulverize through a 100-mesh sieve;
[0070] Step 2: Mix metformin hydrochloride, sitagliptin phosphate monohydrate an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com