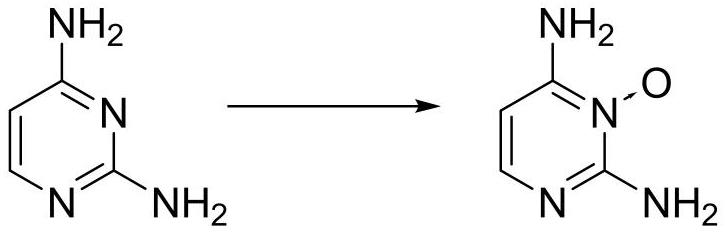
Preparation method of 2, 4-diaminopyrimidine-3-oxide
A technology of diaminopyrimidine and oxide, which is applied in the field of synthesis of 2,4-diaminopyrimidine-3-oxide, and can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
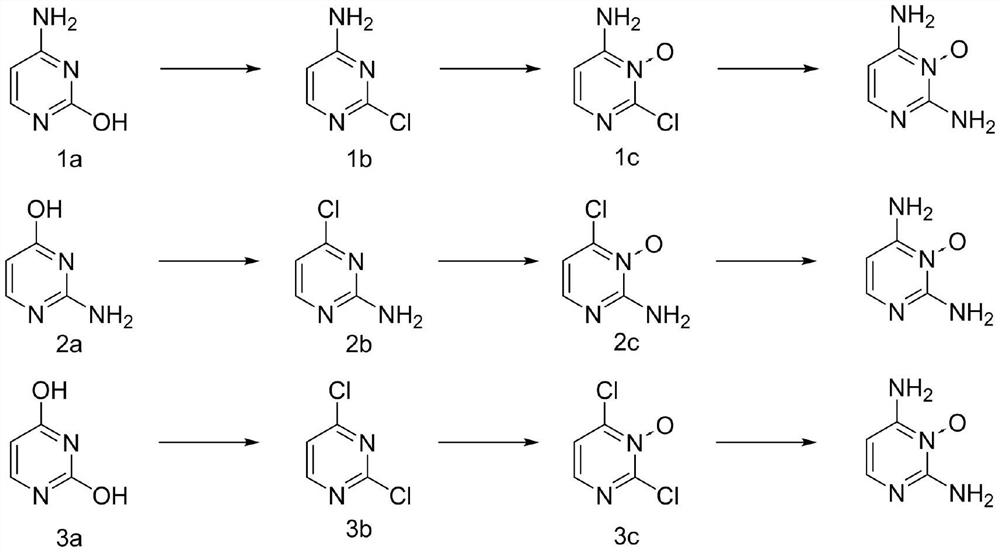
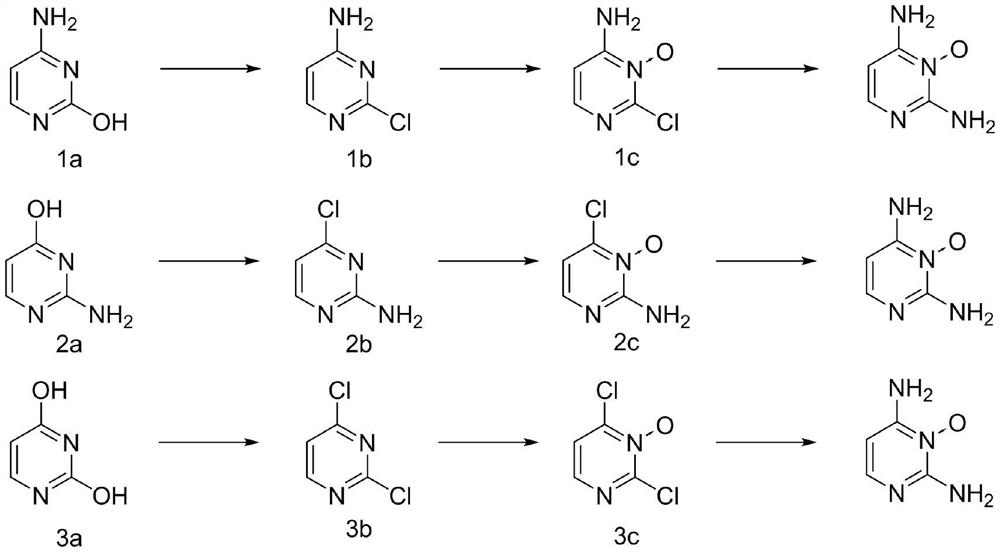
[0031] Put 22.2g (0.2mol) of cytosine, 61.3g (0.4mol, 2eq) of phosphorus oxychloride and 200mL of acetonitrile into the reaction kettle. Raise the temperature to 35°C, add 40.5g (0.4mol, 2eq) of triethylamine dropwise, raise the temperature to 80-82°C and stir for 4 hours after the dropwise addition, take a sample by HPLC to detect that the remaining cytosine of the raw material is less than 0.3%, lower the temperature, and concentrate under reduced pressure to remove For most of the acetonitrile and phosphorus oxychloride, put the reaction solution into 10°C water to quench, adjust the pH to 8-9 with 20% aqueous sodium carbonate solution, add dichloromethane for extraction, concentrate, add n-heptane for beating, and obtain 4- Amino-2-chloropyrimidine 23.2g, yield 89.5%, HPLC 98.9%. 1 HNMR (400MHz, CDCl 3 ):7.80-7.78(m,1H),6.93(s,2H),6.14-6.12(m,1H).
Embodiment 2
[0033]
[0034] 22.2 g (0.2 mol) of isocytosine and 200 mL of toluene were put into the reactor. Raise the temperature to 55°C, add 62.5g (0.3mol, 1.5eq) of phosphorus pentachloride in batches, raise the temperature to 102-108°C and stir for 6 hours, take a sample by HPLC to detect that the remaining cytosine of the raw material is less than 0.2%, cool down and concentrate under reduced pressure to remove most of it Toluene, put the reaction solution into 10°C water to quench, use 20% sodium carbonate aqueous solution to adjust pH=8~9, add dichloromethane to extract, concentrate, add n-heptane for beating, and obtain 2-amino-4-chloropyrimidine 24.1 g, yield 93.1%, HPLC 95.4%. 1 HNMR (400MHz, CDCl 3 ):8.70-8.68(m,1H),6.90-6.88(m,1H),6.33(s,2H).
Embodiment 3
[0036]
[0037] Put 22.4g (0.2mol) of uracil and 153.3g (1.0mol, 5eq) of phosphorus oxychloride into the reactor. Raise the temperature to 105-108°C and stir the reaction for 8 hours, sample HPLC to detect the remaining 0.12% of the raw material cytosine, cool down and concentrate under reduced pressure to remove most of the phosphorus oxychloride, put the reaction solution into 10°C water to quench, and use 10% bicarbonate Potassium aqueous solution was used to adjust the pH to 8-9, dichloromethane was added for extraction, concentrated, and n-heptane was added for slurry to obtain 28.5 g of 2,4-dichloropyrimidine with a yield of 95.7% and HPLC of 99.1%. 1 HNMR (400MHz, CDCl 3 ):8.83-8.80(m,1H),7.38-7.36(m,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com