Triazole analogue compound as well as preparation method and application thereof
A technology of analogs and triazoles, which is applied in the field of preparation of triazole analogs, can solve the problems of unstable compounds, high toxicity of raw materials, cumbersome post-processing, etc., and achieve the effects of low toxicity, economical reliability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
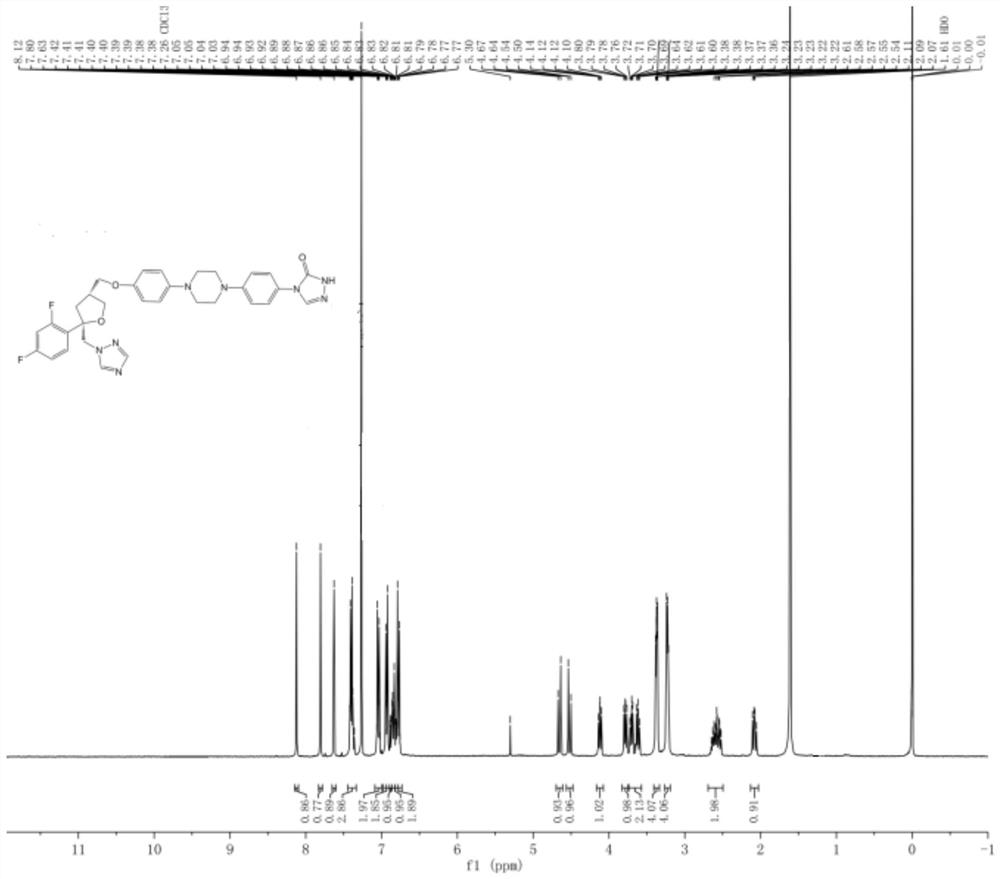
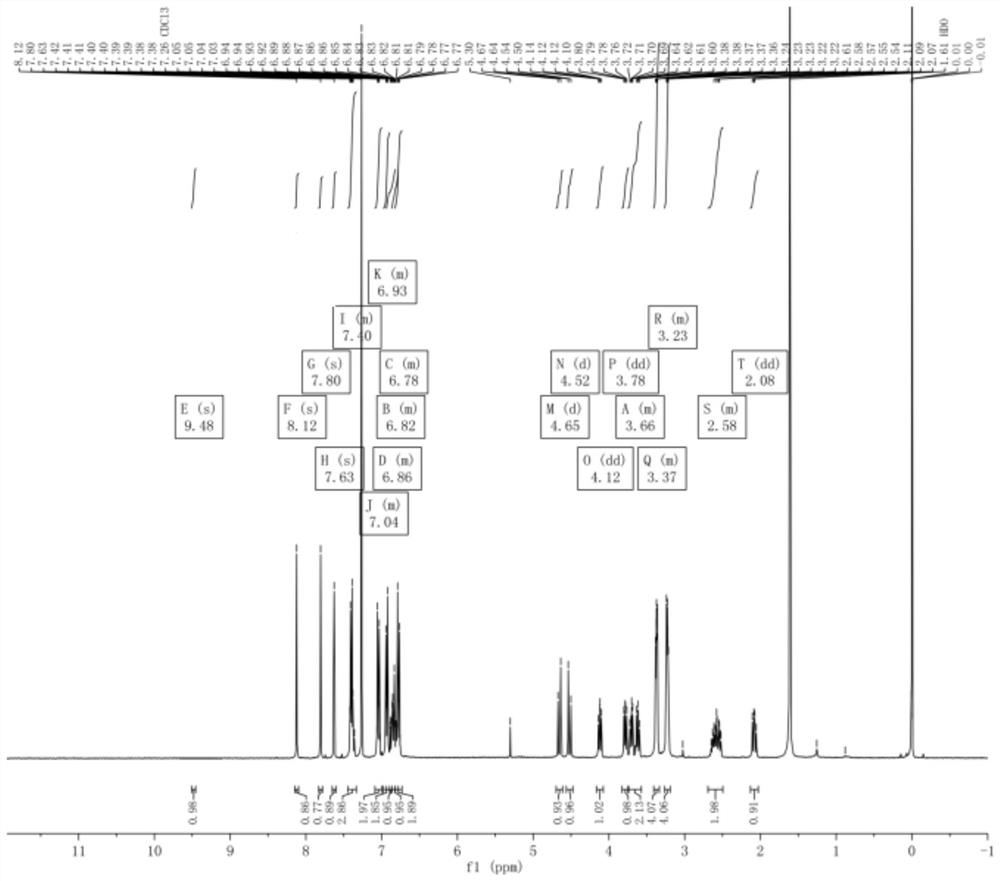
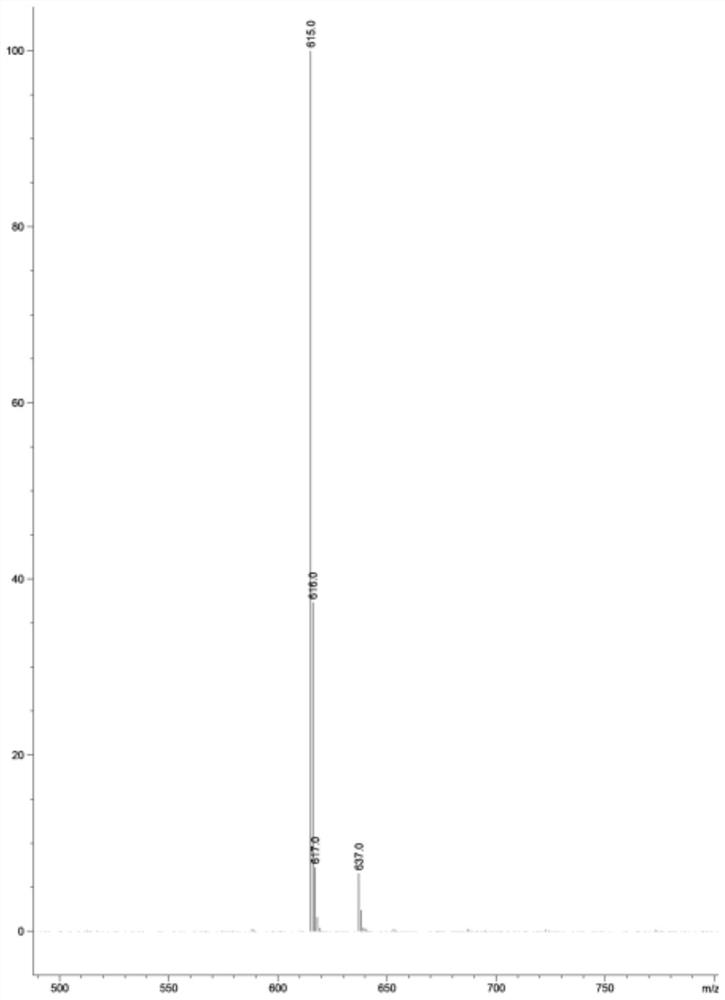
[0052] Add 5.0g of compound 1, 0.5g of compound 2, 0.46g of DMAP, 0.77g of imidazole, 6ml of DME and stir to dissolve, react at 25°C for 24h, drop the reaction solution into 50ml of water, extract with 100ml of dichloromethane, and concentrate under reduced pressure to obtain dark brown Oily liquid, column chromatography (using 18*160mm glass chromatography column, 300-400 mesh silica gel, petroleum ether and ethyl acetate volume ratio of 6:1 mixed solvent as eluent) was purified for 3 times to obtain a white solid (compound 3) 3.8g, yield 82.9%, purity 98.1%.
Embodiment 2
[0054] Add 5.0g of compound 1, 0.5g of compound 2, 0.46g of DMAP, 0.77g of imidazole, 6ml of MeOH and stir to dissolve, react at 25°C for 24h, drop the reaction liquid into 25ml of water, extract with 100ml of dichloromethane, and concentrate under reduced pressure to obtain dark brown Oily liquid, column chromatography (using 18*160mm glass chromatography column, 300-400 mesh silica gel, petroleum ether and ethyl acetate volume ratio of 6:1 mixed solvent as eluent) was purified for 3 times to obtain a white solid (compound 3) 3.82g, yield 83.1%, purity 98.5%.
Embodiment 3
[0056] Add 5.0g of compound 1, 0.5g of compound 2, 0.46g of DMAP, 0.77g of triethylamine, 6ml of MeOH and stir to dissolve, react at 25°C for 24h, drop the reaction liquid into 25ml of water, extract with 100ml of dichloromethane, and concentrate under reduced pressure to obtain Dark brown oily liquid, purified by column chromatography (using 18*160mm glass chromatography column, 300-400 mesh silica gel, petroleum ether and ethyl acetate with a volume ratio of 6:1 as eluent) 3 times to obtain a white solid (Compound 3) 3.48g, yield 75.8%, purity 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com