Geniposide derivative with xanthine oxidase inhibitory activity as well as preparation method and application of geniposide derivative
A technology of xanthine oxidase and geniposide, which is applied in the field of geniposide derivatives and preparation, can solve problems such as non-use, achieve the effect of improving biological activity, low raw material price, and inhibiting xanthine oxidase activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
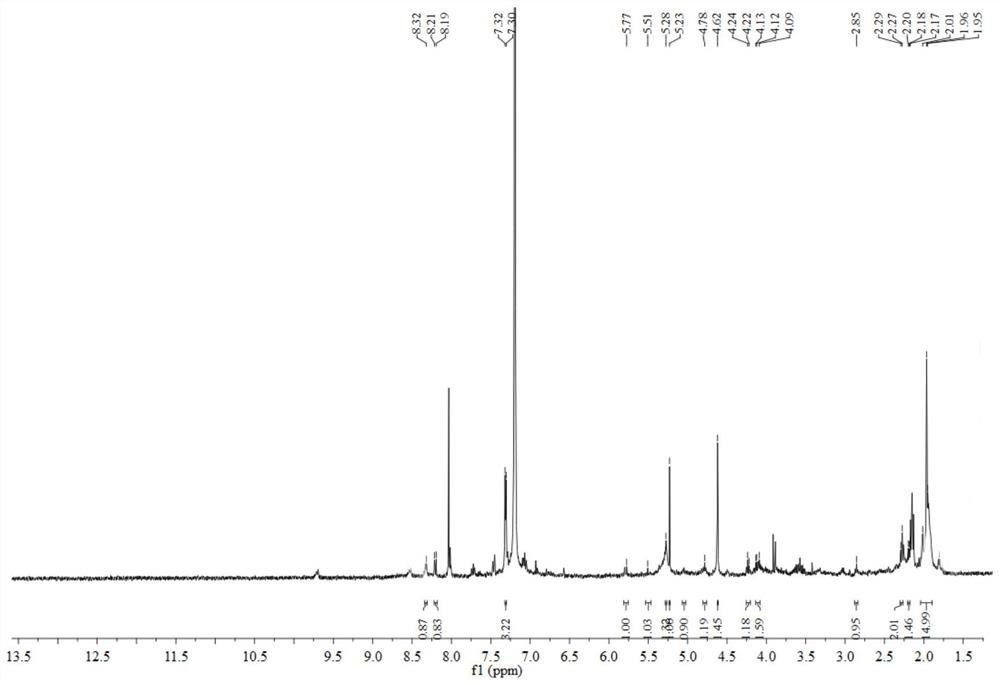
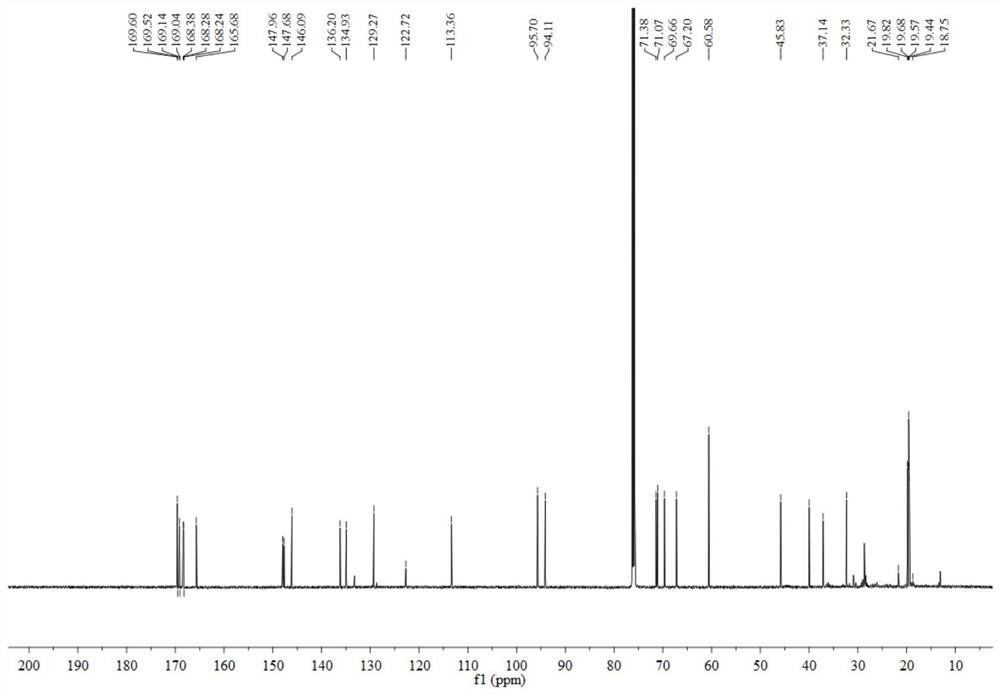
[0035] The preparation method of geniposide derivatives with xanthine oxidase inhibitory activity: First, 200 mg of geniposide (about 0.51 mmol) was dissolved in 3 mL of dichloromethane, placed in a two-necked flask, and added to the flask 10ml 4% NaOH solution, temperature controlled to 65°C. After reacting for 3 h, distill under reduced pressure and spin dry the water. Add 5ml of acetic anhydride and 1ml of triethylamine, and stir overnight at room temperature. After the reaction, distill under reduced pressure, spin the solution to dryness, add 0.12 mml oxalyl chloride, and react at room temperature for 3 h. After the reaction, distill under reduced pressure, spin the solution to dryness, add 50.7 mg (about 0.37 mmol) of 4-mercaptopyridine under ice bath, react at room temperature, and track the reaction by TLC. After the reaction, the reaction solution was concentrated and purified through a neutral alumina column. The eluent was petroleum ether: ethyl acetate (8:1, 5:1,...
Embodiment 2
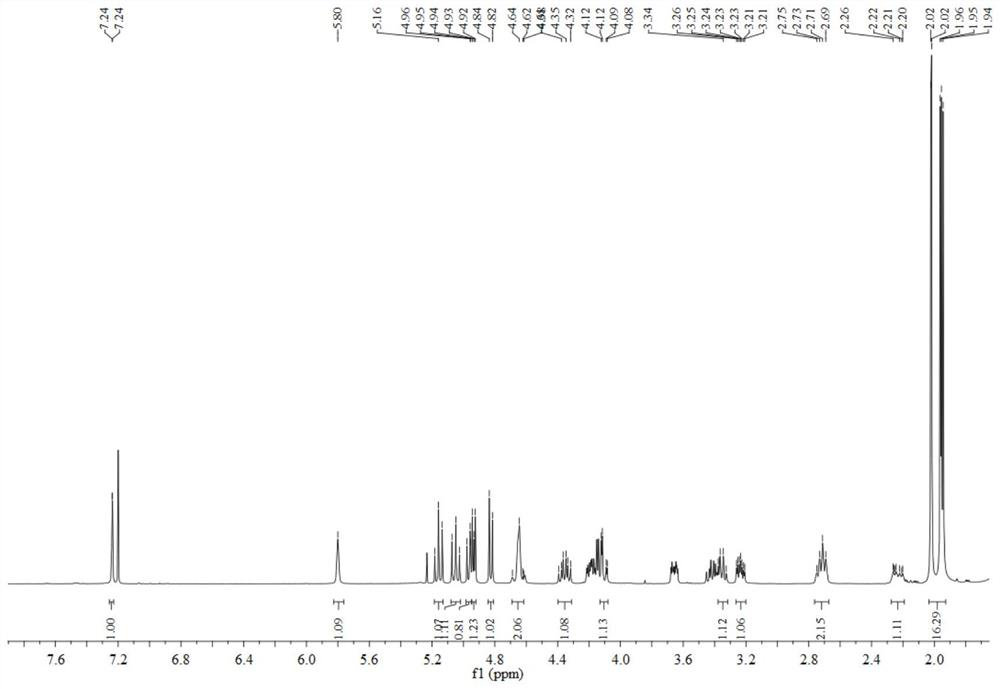
[0041]The preparation method of geniposide derivatives with xanthine oxidase inhibitory activity, first dissolve 200 mg geniposide (about 0.51 mmol) in 3 mL methylene chloride, place in a two-necked flask, add 10ml 4% NaOH solution, temperature controlled to 65°C. After reacting for 3 h, distill under reduced pressure and spin dry the water. Add 5ml of acetic anhydride and 1ml of triethylamine, and stir overnight at room temperature. After the reaction, distill under reduced pressure, spin the solution to dryness, add 0.12 mmol oxalyl chloride, and react at room temperature for 3 h. After the reaction, distill under reduced pressure, spin the solution to dryness, add 41.5 mg (about 0.37 mmol) of 2-mercaptothiazoline under ice bath, react at room temperature, and track the reaction by TLC. After the reaction, the reaction solution was concentrated and purified through a neutral alumina column. The eluent was petroleum ether: ethyl acetate (8:1, 5:1, 3:1, 2:1, 1:1), and separa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com