Application of quercetin dimer flavone for preparing anti-oxidation medicines
A technology of quercetin and dimer, which is applied in the field of medicine, can solve the problem that quercetin dimer protects PC12 cells that have not been seen, and achieves low pollution, large-scale production, and powerful removal The effect of free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
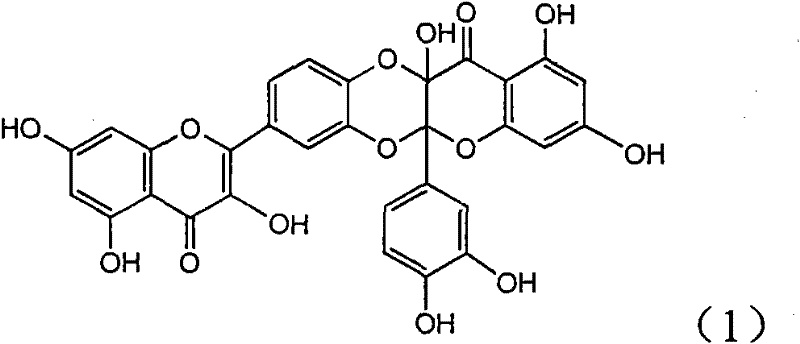
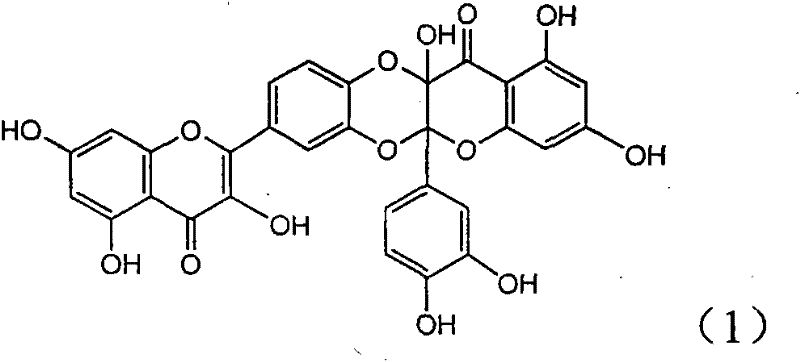
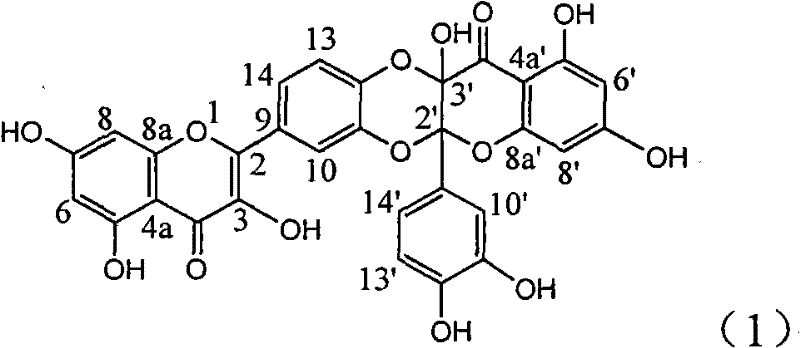
[0017] Example 1 : the preparation of compound (1)
[0018]
[0019] Add 343 milligrams of quercetin (prepared by ourselves, HPLC detection purity 98%), 550 milligrams of silver carbonates in the dry reaction bottle, add 20 milliliters of dry acetone and 60 milliliters of anhydrous benzene solution under nitrogen protection, in Stir between 55-60°C for 20 hours. The mixture was left standing, and insoluble matter was filtered off. The mother liquor was concentrated under reduced pressure to obtain a yellow solid. It was chromatographed on a 200-300 mesh silica gel column and eluted with chloroform / methanol (20:1). 182mg of yellow solid was obtained. R f (chloroform:methanol=10:1)=0.18; infrared IR (KBr tablet) cm -1 : 3195, 1689, 1647, 1588, 1495; H NMR 1 H NMR (400MHz, deuterated acetone): 6.05 (doublet, J=2.0Hz, 1H, H-6'), 6.10 (doublet, J=2.0Hz, 1H, H-8'), 6.28 (doublet , J=2.0Hz, 1H, H-6), 6.62 (doublet, J=2.0Hz, 1H, H-8), 6.81 (doublet, J=8.4Hz, 1H, H-13), 7.16...
Embodiment 2
[0026] Example 2 : activity test of formula (1) compound in vitro scavenging superoxide anion free radical
[0027] 2.1 Experimental materials and samples:
[0028] 2.1.1 Experimental reagents:
[0029] 2.1.1.1 Phenazine methosulfate (PMS), nitroblue tetrazolium (NBT), and phenanthrozine (ferrozine) were purchased from Sigma;
[0030] 2.1.1.2 Quercetin (quercetin) was provided by the Laboratory of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University (purity: 99%);
[0031] 2.1.1.3 Tris base, DMEM medium was purchased from Gibco;
[0032] 2.1.1.4 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and NADH (reduced coenzyme I) were purchased from Amresco;
[0033] 2.1.1.5 Other reagents are domestic analytical reagents, purchased from Hangzhou Huadong Pharmaceutical Reagent Co., Ltd.
[0034] 2.1.2 Instruments:
[0035] 2.1.2.1 Microplate reader: Synergy-HT type, BIO-TEK company;
[0036] 2.1.2.2 Vertical automatic el...
Embodiment 3
[0048] Example 3 : formula (1) compound to hydrogen peroxide H 2 o 2 Protective activity test of induced PC12 cell injury
[0049] 3.1 Experimental materials and samples:
[0050] 3.1.1 Cells: Rat adrenal pheochromoma cells (PC12) were purchased from Shanghai Institute of Cells, Chinese Academy of Sciences.
[0051] 3.1.2 Experimental reagents:
[0052] 3.1.2.1 Hydrogen peroxide (H 2 o 2 ), nitrobluetetrazolium (NBT), phenanthrozine (ferrozine) were purchased from Sigma Company;
[0053] 3.1.2.2 Quercetin (quercetin) was provided by the Laboratory of Traditional Chinese Medicine and Natural Medicine, School of Pharmacy, Zhejiang University (purity: 99%);
[0054] 3.1.2.3 Tris base, DMEM medium was purchased from Gibco;
[0055] 3.1.2.4 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amresco;
[0056] 3.1.2.5 Calf serum was purchased from Hangzhou Sijiqing Bioengineering Materials Co., Ltd.;
[0057] 3.1.2.6 Penicillin and strept...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com