Preparation method of potential anti-new coronavirus drug monaprevir
A virus, a potential technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of long reaction time and low reaction yield, and achieve simple operation, high quality purity, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] A preparation method of potential anti-new coronavirus drug monaprevir, which comprises the following process steps:
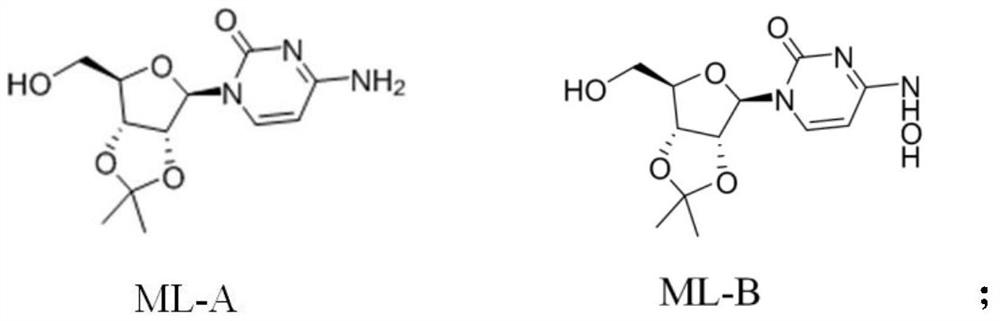
[0023] Step (1) Synthesis of intermediate ML-B: Dissolving material ML-A in an organic solvent, reacting with hydroxylamine salt to obtain intermediate ML-B, the structural formulas of said ML-A and intermediate ML-B are respectively:
[0024]
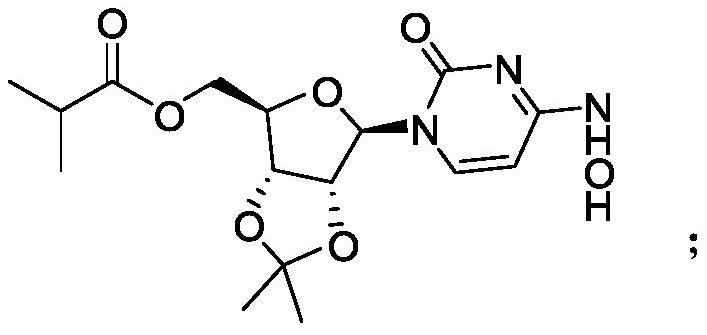
[0025] The synthesis of step (2) intermediate ML-C: the intermediate ML-B obtained in step (1) reacts with acid anhydride to obtain intermediate ML-C through transesterification, and the structural formula of said intermediate ML-C is:
[0026]
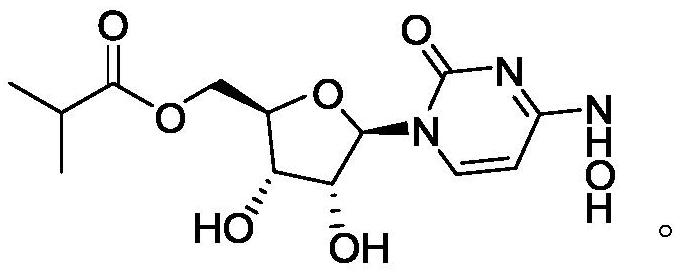
[0027] The synthesis of step (3) monaprevir: the intermediate ML-C deprotection reaction of step (2) gained obtains monaprevir, and the structure of described monaprevir is:
[0028]
[0029] Concrete synthetic route of the present invention is as follows:
[0030]
[0031] In step (1), the material ML-A is dissolved in an organic solvent, and reacts with h...
Embodiment 1
[0050] 1.1 Synthesis of intermediate ML-B
[0051] In a 2L three-necked flask, add ML-A (28.3g, 0.10mol), DMF (500ml), HOBt (14.8g, 0.11mol), hydroxylamine hydrochloride (8.3g, 0.13mol) and stir to dissolve, heat up to 75- 80 ° C, stirring at this temperature for 10-12h. After the reaction was completed, 500ml of water was added and stirred for 15min. Extracted 2 times with 300ml*2 acetic acid, the obtained organic layer solution was washed 2 times with 300ml*2 water, and the organic layer was dried and concentrated to obtain 28.1g of solid ML-B with a yield of 93.8%.
[0052] NMR analysis:
[0053] ML-B:1H NMR(400MHz,DMSO)δ10.05(s,1H),9.61(s,1H),7.00(d,J=8.2Hz,1H),5.80(d,J=3.1Hz,1H) ,5.60(d,J=8.1Hz,1H),5.04(t,J=5.1Hz,1H), 4.84(dd,J=6.4,3.1Hz,1H),4.74(dd,J=6.3,3.8Hz, 1H), 3.99(dd, J=8.1, 4.2Hz, 1H), 3.67–3.51(m, 2H), 1.51(s, 3H), 1.31(s, 3H).
[0054] ESI-MS: m / z 322.38[M+Na] + .
[0055] 1.2 Synthesis of intermediate ML-C
[0056] In a 2L three-neck flask, add solid ML-...
Embodiment 2
[0066] 2.1 Synthesis of intermediate ML-B
[0067] In a 2L three-necked flask, add ML-A (28.3g, 0.10mol), DMSO (500ml), BOP (53.1g, 0.12mol), hydroxylamine sulfate (24.6g, 0.15mol) and stir to dissolve, heat up to 85- 90°C, stirring at this temperature for 11-13h. After the reaction was completed, 500ml of water was added and stirred for 15min. Extracted 2 times with 300ml*2 acetic acid, the obtained organic layer solution was washed 2 times with 300ml*2 water, and the organic layer was dried and concentrated to obtain 26.8g of solid ML-B with a yield of 89.8%.
[0068] 2.2 Synthesis of intermediate ML-C
[0069] In a 2L three-necked flask, add solid ML-B (29.9g, 0.1mol), anhydrous DMF 300ml and stir to clarify, then add DCC (6.18g, 0.03mol), DMAP (3.66g, 0.03mol), and then add TEA (25.3g, 0.25mol) was stirred for 30min, then the temperature was controlled to 20-25°C, and isobutyric anhydride (20.56g, 0.13mol) was added slowly at this temperature, and the reaction was conti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com